Production method for high-purity ferric phosphate
A production method and high-purity phosphoric acid technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of high impurity content and uneven iron phosphate impurity content, and achieve the effect of overcoming the low purity of the product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
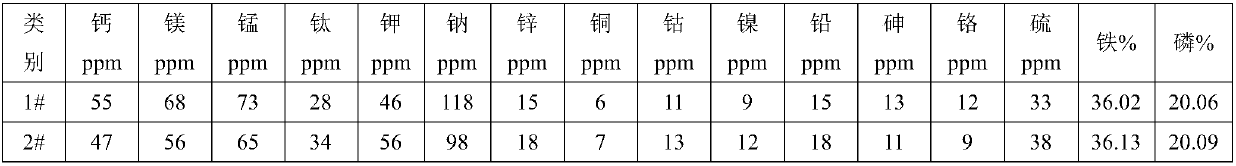
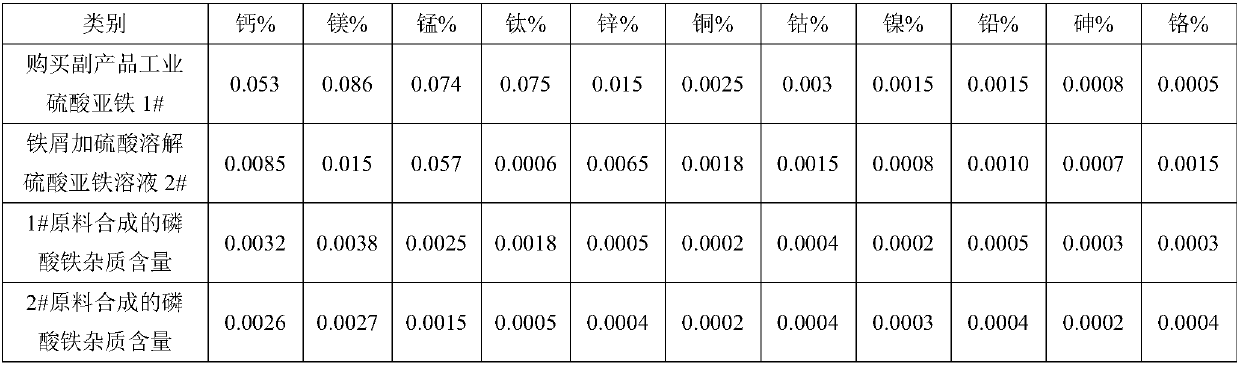
[0043]Add 1000L of pure water to the reaction kettle, add 300kg of industrial grade ferrous sulfate heptahydrate, raise the temperature to 60°C, add 2.5kg of reduced iron powder, hydrolyze for 3 hours, adjust the pH to 4.5-5.0, and then add 0.5 kg SDD (Sodium Dimethicone), add 2L of 0.05% cationic polyacrylamide after reacting for 1 hour, stir well and filter; add 2L of 55% hydrofluoric acid dropwise in the filtrate, stir and keep warm at 60°C for 2 hours and filter; Add 4 liters of 85% phosphoric acid under stirring, stir and react for 2 hours, let it stand for 2 hours, and filter to obtain a pure ferrous sulfate solution; add 180L of 27.5% hydrogen peroxide to the above ferrous sulfate solution to ensure divalent After the iron has been completely converted into ferric iron, add 2kg PEG to the reactor and stir well, then add dropwise ammonium dihydrogen phosphate solution (150kg ammonium dihydrogen phosphate dissolved in 400L pure water), and add phosphoric acid in an appropr...
Embodiment 2
[0048] Add 1200L of pure water to the reaction kettle, add 450kg of industrial grade ferrous sulfate heptahydrate, raise the temperature to 60°C, add 2.5kg of reduced iron powder, hydrolyze for 3 hours, adjust the pH to 4.5-5.0, and then add 0.5kg After SDD (Sodium Dimethicone) was reacted for 0.5 hours, add 3L of 0.05% flocculant, stir well and filter; add 2L of 55% hydrofluoric acid dropwise in the filtrate, stir and keep warm at 60°C for 2 hours to react and filter; Add 4 liters of 85% phosphoric acid, stir and react for 2 hours and then stand still for 2 hours, filter to obtain pure ferrous sulfate solution; add 300L of 27.5% hydrogen peroxide to the above ferrous sulfate solution to ensure that ferrous iron has all been converted into trivalent iron After valence of iron, add 2.5kgPEG to the reaction kettle and stir well, then add dropwise ammonium dihydrogen phosphate solution (250kg ammonium dihydrogen phosphate dissolved in 600L pure water), add phosphoric acid in an ap...
Embodiment 3
[0052] Add 2 mol / L 1500 liters of iron filings and sulfuric acid to the reaction kettle to form a ferrous sulfate solution, raise the temperature to 60°C, add 3 kg of reduced iron powder, hydrolyze for 3 hours, adjust the pH to 4.5-5.0, and then add 1.5 kg SDD (Sodium Dimethicone), after reacting for 0.5 hours, add 4L of 0.05% flocculant, stir well and filter; add 5L of 55% hydrofluoric acid dropwise in the filtrate, stir and keep warm at 60°C for 2 hours and filter; Add 6 liters of 85% phosphoric acid under stirring, stir for 2 hours and then stand still for 2 hours, filter to obtain a pure ferrous sulfate solution; add 500L of 27.5% hydrogen peroxide to the above ferrous sulfate solution to ensure that the ferrous iron has been completely converted After making ferric iron, add 4kg PEG to the reaction kettle and stir well, then add dropwise ammonium dihydrogen phosphate solution (450kg ammonium dihydrogen phosphate dissolved in 1000L pure water), add phosphoric acid in an app...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com