Non-ferrous metal smelting high-arsenic contaminated acid arsenic fixing process
A high-arsenic polluted acid, non-ferrous metal technology, applied in water/sewage treatment, metallurgical wastewater treatment, neutralized water/sewage treatment, etc. Efficiency, complex process and other problems, to achieve the effect of reducing the amount of solid waste, low risk of secondary pollution, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
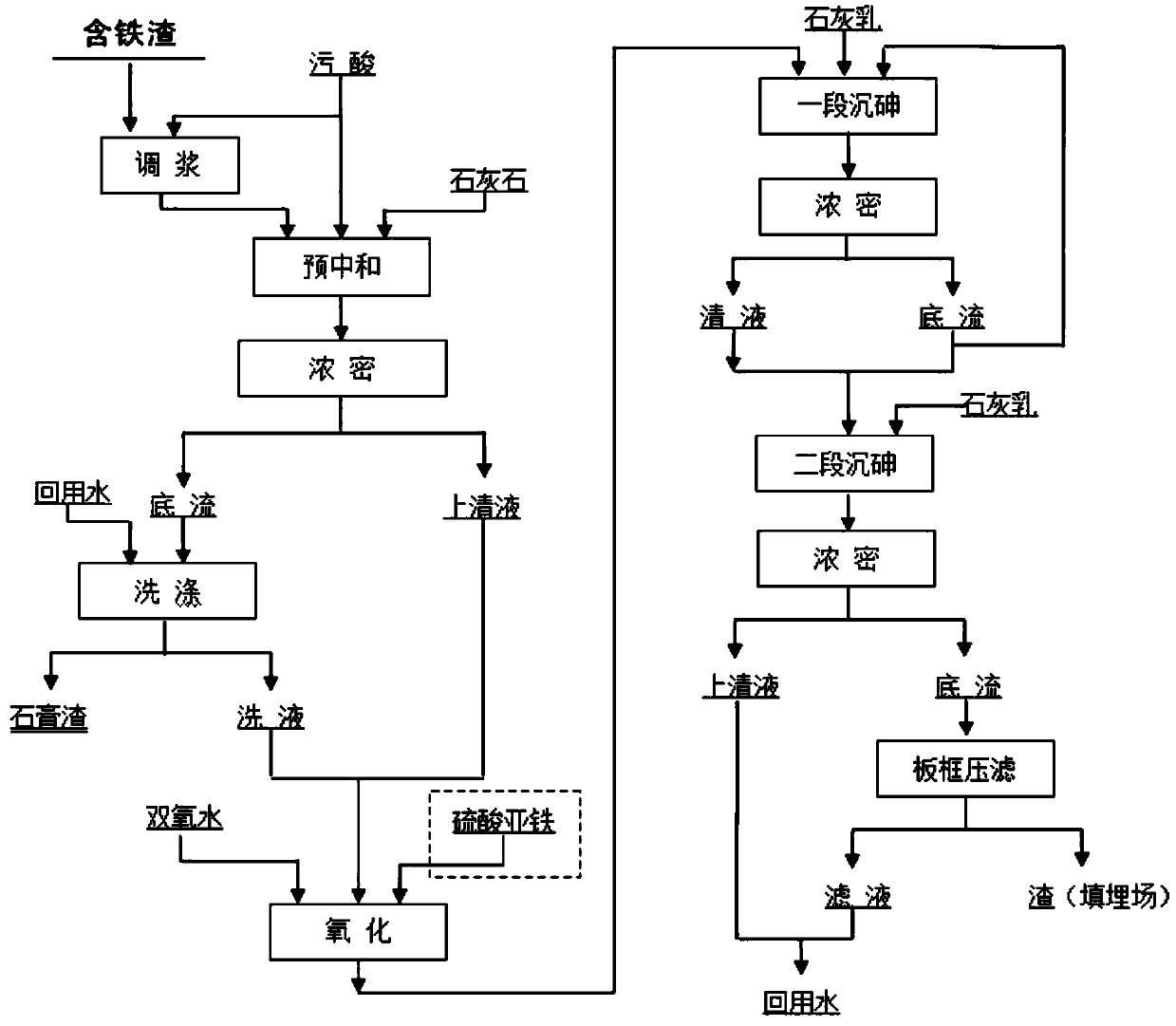
[0035] (1) Pre-neutralization
[0036] Neutralize slag (Fe 10.18%, Ca 23.90%, As 0.048% and Cu 0.10%) in acid mine water with arsenic-containing polluted acid (H 2 SO 4 93.1g / L, As 9.1g / L, potential 364mv), add according to Fe / As molar ratio 1.2, stir and dissolve for 1 hour, monitor the pH value online, add calcium carbonate as needed to adjust the pH value to 0.9, continue stirring for 30 minutes, solid-liquid separation;
[0037] (2) oxidation
[0038] The pre-neutralized filtrate is placed in a reactor with mechanical stirring, and ferrous sulfate is added to make the Fe / As molar ratio 1.2, the heating temperature is controlled at 70°C, hydrogen peroxide is added in batches, and the process titration monitors the change of trivalent arsenic content and monitors the oxidation Reduction potential, control the terminal potential 650mV to ensure that trivalent arsenic is completely oxidized to pentavalent arsenic;
[0039] (3) Precipitation of arsenic
[0040] One-stage a...
Embodiment 2
[0044] (1) Pre-neutralization
[0045] Copper smelting flotation iron-containing tailings (Fe 44.7%, Si14.5%, Ca 1.32%, As 0.04% and Cu0.2%) and arsenic-containing acid (H 2 SO 4 160g / L, As 25.7g / L, potential 455mv), add according to Fe / As molar ratio 1.5, stir and dissolve for 2 hours, monitor the pH value online, add calcium carbonate as needed to adjust the pH value to 1.1, continue stirring for 30min, solidify liquid separation;
[0046] (2) oxidation
[0047] The pre-neutralized filtrate is placed in a reactor with mechanical stirring, and ferrous sulfate is added to make the Fe / As molar ratio 1.1, the heating temperature is controlled at 50°C, hydrogen peroxide is added in batches, and the process titration monitors the change of trivalent arsenic content and monitors the oxidation Reduction potential, control the terminal potential 600mV to ensure that trivalent arsenic is completely oxidized to pentavalent arsenic;
[0048] (3) Precipitation of arsenic
[0049] On...
Embodiment 3
[0053] (1) Pre-neutralization
[0054] Arsenic acid (H 2 SO 4 220g / L, As 15.3g / L, potential 385mv), add calcium carbonate, monitor the pH value online to adjust the pH value to 0.9, continue stirring for 30min, and separate the solid and liquid;
[0055] (2) oxidation
[0056] The pre-neutralization filtrate is placed in a reactor with mechanical stirring, and ferric sulfate is added to make the Fe / As molar ratio 1.2, and the heating temperature is controlled at 60°C. Hydrogen peroxide is added in batches, and the process titration monitors the change of trivalent arsenic content and the oxidation-reduction potential , control the terminal potential of 570mV to ensure that trivalent arsenic is completely oxidized to pentavalent arsenic;
[0057] (3) Precipitation of arsenic
[0058] One-stage arsenic precipitation: transfer the oxidized solution to the arsenic precipitation device with mechanical agitation, raise the temperature to 90°C, add 200g / L of seed crystals, add li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com