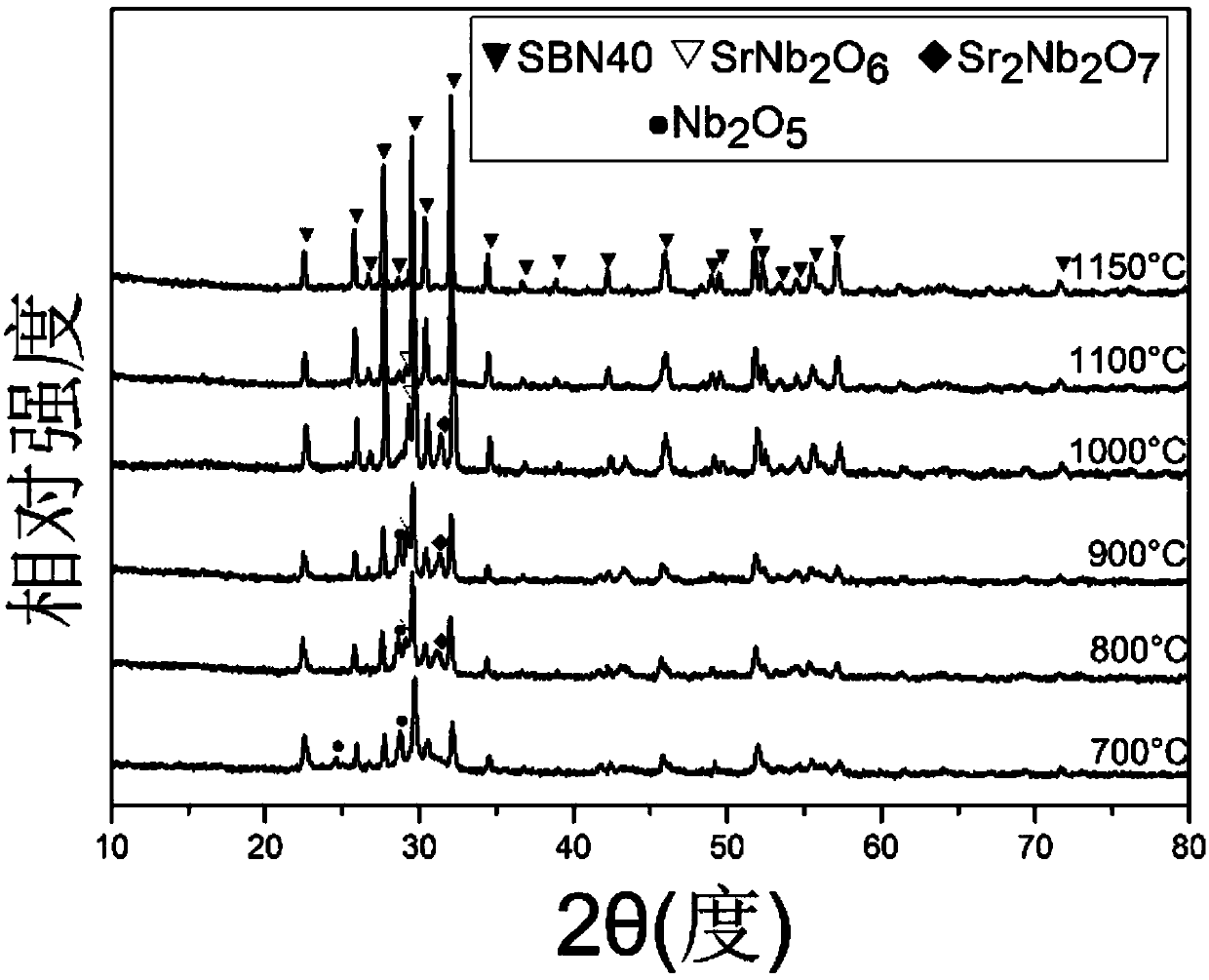
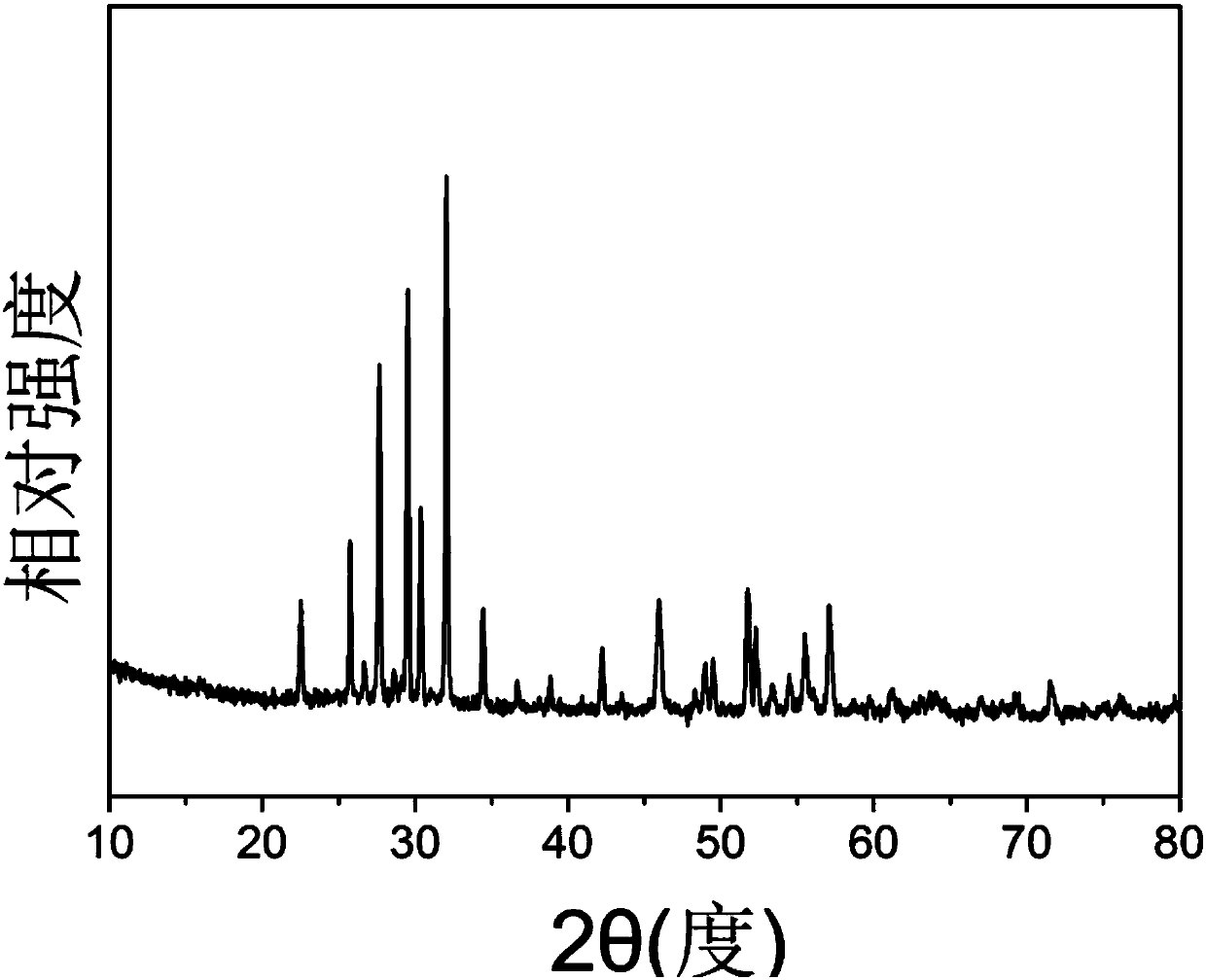
Method for preparing strontium barium niobate nano-powder through two-step coprecipitation technology
A technology of barium strontium niobate and co-precipitation method, which is applied in chemical instruments and methods, niobium compounds, inorganic chemistry, etc., can solve problems such as potential safety hazards, reduce preparation costs, avoid toxic and harmful reagents, and powder microscopic morphology controllable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] To prepare 0.025mol Sr 0.4 Ba 0.6 Nb 2 o 6 Taking nano powder as an example, the required raw materials are 21.16 grams of niobium oxalate, 3.87 grams of barium acetate, 2.13 grams of strontium nitrate, 100ml of 3mol / L ammonium carbonate solution, and 400ml of ammonia water. The specific operation is as follows:
[0025] 1. Prepare 3mol / L (NH 4 ) 2 CO 3 solution;
[0026] 2. Dissolve 21.16 grams of niobium oxalate, 3.87 grams of barium acetate and 2.13 grams of strontium nitrate in 300ml, 20ml and 20ml of deionized water respectively at room temperature, and keep stirring to obtain a transparent and clear aqueous solution of niobium oxalate, barium acetate and strontium nitrate;
[0027] 3. Take 100ml of the (NH 4 ) 2 CO 3 In the solution, after mixing the barium acetate and strontium nitrate solution prepared in step 2, add the (NH 4 ) 2 CO 3 in solution;
[0028] 4. Add the niobium oxalate solution prepared in step 2 dropwise to the mixed solution prepar...
Embodiment 2
[0037] To prepare 0.025mol Sr 0.5 Ba 0.5 Nb 2 o 6 Taking nano powder as an example, the required raw materials are 21.16 grams of niobium oxalate, 3.23 grams of barium acetate, 2.66 grams of strontium nitrate, 100ml of 3mol / L ammonium carbonate solution, and 400ml of ammonia water. The specific operation is as follows:
[0038] 1. Prepare 3mol / L (NH 4 ) 2 CO 3 solution;
[0039] 2. Dissolve 21.16 grams of niobium oxalate, 3.23 grams of barium acetate and 2.66 grams of strontium nitrate in 300ml, 20ml, and 20ml of deionized water respectively at room temperature, and keep stirring to obtain a transparent and clear aqueous solution of niobium oxalate, barium acetate and strontium nitrate;
[0040] 3. Take 100ml of the (NH 4 ) 2 CO 3 In the solution, after mixing the barium acetate and strontium nitrate solution prepared in step 2, add the (NH 4 ) 2 CO 3 in solution;
[0041] 4. Add the niobium oxalate solution prepared in step 2 dropwise to the mixed solution prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com