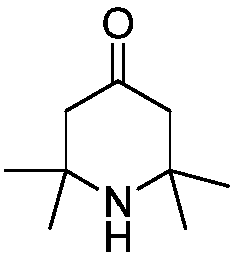
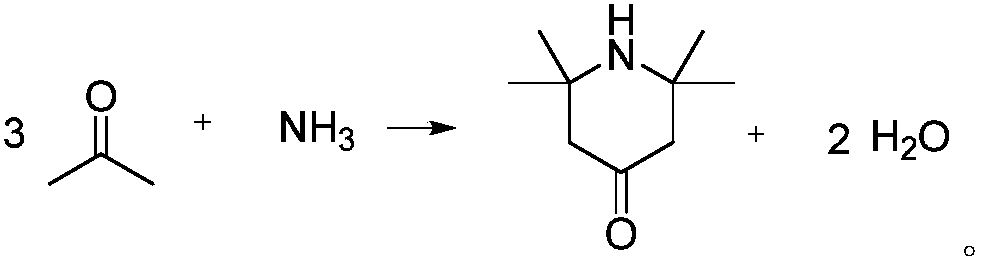
2,2,6,6-tetramethylpiperidone synthesis method for setting end-point determination mode
A technology of tetramethylpiperidone and synthesis method, applied in the direction of organic chemistry, etc., to achieve the effects of less side reactions, high yield, and reducing the amount of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, add acetone 87g (1.5mol), 8g (0.1mol) ammonium nitrate in the autoclave of 250mL; Pass into liquid ammonia 8.5g (0.5mol), heat up to 60 ℃ under stirring; Continuous detection in the reaction process When the pH value of the reaction system does not change within 30 minutes, the heating can be stopped, thereby terminating the reaction. At this time, the reaction time is about 4 hours, and the pressure of the autoclave is about 0.1 MPa; the concentration of the product in the GC is 48%.
[0024] After the reaction is completed, the reaction solution is suction-filtered in a Buchner funnel first, and the obtained filtrate is roughly steamed under normal pressure, and the obtained light components are recovered and used mechanically (that is, acetone is recovered by distillation); Sodium hydroxide is used to absorb the water generated by the reaction. The mass ratio of sodium hydroxide to the crude steamed heavy component (that is, the distillation substrate) ...
Embodiment 2
[0025] Embodiment 2, the reaction temperature of embodiment 1 is changed from 60 ℃ to 65 ℃, and the rest are equal to embodiment 1.
[0026] The final yield of 2,2,6,6-tetramethylpiperidone was 81.8%.
Embodiment 3
[0027] Embodiment 3, change the consumption of raw material into: acetone 87g (1.5mol), 4g (0.05mol) ammonium nitrate; Feed liquid ammonia 8.5g (0.5mol), all the other are equal to embodiment 1.
[0028] The final yield of 2,2,6,6-tetramethylpiperidone was 80.5%.
[0029] Embodiment 3, change the consumption of raw material into: acetone 87g (1.5mol), 12g (0.15mol) ammonium nitrate; Feed liquid ammonia 8.5g (0.5mol), all the other are equal to embodiment 1.
[0030] The final yield of 2,2,6,6-tetramethylpiperidone was 81.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com