Preparation method of alkene epoxidation catalyst as well as catalyst prepared thereby
An epoxidation and catalyst technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, etc., can solve the complicated preparation process, long reaction time, harsh conditions, etc. problems, to achieve the effect of improving epoxidation selectivity, improving carbon deposition resistance, and reducing surface acidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
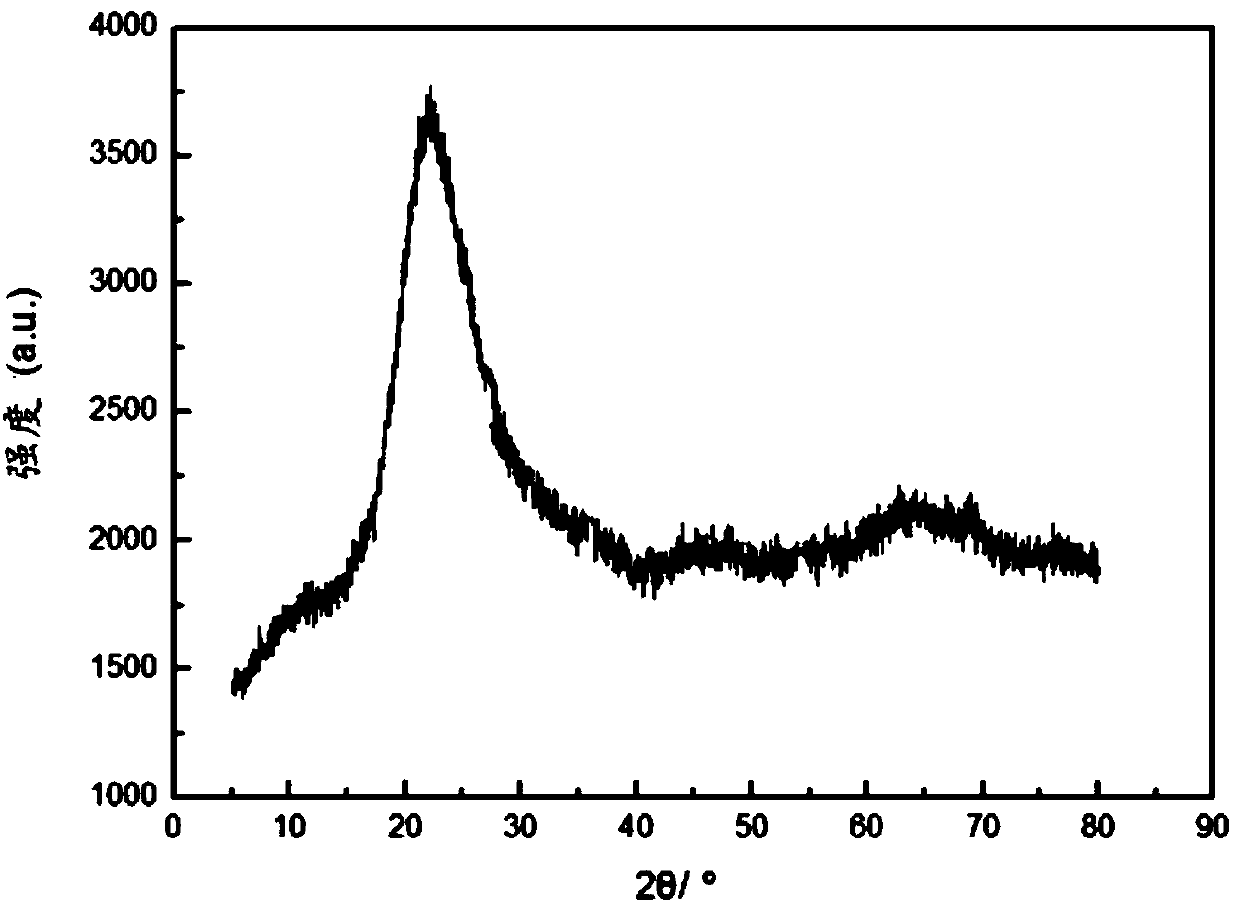
[0056] The structure of the catalyst prepared in Example 1 was determined by X-ray diffractometer. The instrument is produced by PANalytical Company, the model is X’pert 3 powder.
[0057] 3. Confirmation of alkali metal modification and content
[0058] XRF analysis was carried out on the catalysts prepared in each example and comparative example to confirm whether the modification process of the alkali metal element in the step (3) was realized and the content of the alkali metal element. The instrument is produced by Shimadzu Corporation, Japan, and the model is EDX-LE.
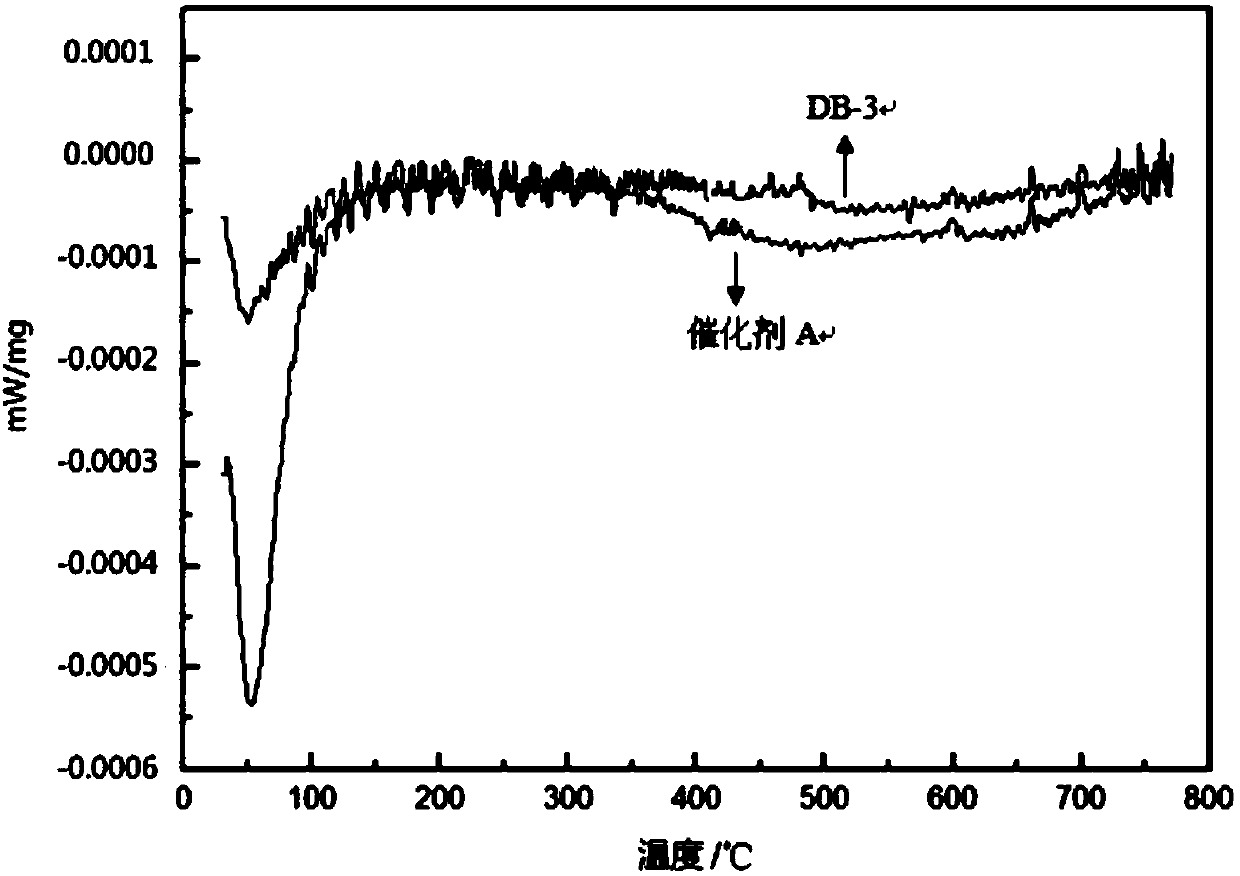
[0059] 4. Silanization modification and confirmation of silyl content
[0060] The catalysts prepared in Example 1 and Comparative Example 3 were thermally analyzed using a scanning calorimeter to obtain a DSC curve, thereby confirming whether the silanization modification process in step (4) was realized. The scanning calorimeter is produced by Mettler-Toledo, the model is DSC1 professional.
[0061]...
Embodiment 2
[0093] Prepare titanium silica gel according to Example 1, the difference is that 20g of ethylenediamine liquid is added during the pore expansion process, and the temperature is treated at 90°C for 10h, then dried at 120°C for 3h, and roasted at 550°C for 3h to obtain a titanium-silicon composite oxide gel. material, the specific surface area determined by BET is 368m 2 / g, the average pore diameter is 10.7nm. Catalyst B was prepared by carrying out alkali metal modification and gas-phase silylation treatment under the same conditions as in Example 1.
Embodiment 3
[0095] Prepare titanium silica gel according to Example 1, the difference is that 20g of high-purity liquid ammonia is added during the pore expansion process, and the temperature is treated at 150°C for 12h, then dried at 160°C for 3h, and roasted at 550°C for 3h to obtain titanium-silicon composite oxide. material, its specific surface area is 322m as determined by BET 2 / g, the average pore diameter is 18.3nm. Catalyst C was prepared by carrying out alkali metal modification and gas-phase silylation treatment under the same conditions as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Average pore size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com