Compounds and their preparation methods and applications
A compound and reaction technology, applied in the fields of peptide preparation, chemical instruments and methods, thioether preparation, etc., can solve the problems of difficulty in obtaining ubiquitin chains and ubiquitin-modified substrates, and achieve high cleavage reaction efficiency and yield. High, easy-to-operate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] In the polypeptide preparation method of the present invention, the partial protein fragment with cysteine can be prepared by recombinant expression, which is simple and convenient. In addition, the inventors have found through extensive experiments that this method is particularly suitable for the preparation of polyubiquitin chains and substrate proteins modified by ubiquitin chains. For example, in the first embodiment of the present invention, the first protein fragment has the amino acid sequence shown in SEQ ID NO: 1, the second protein fragment has the sequence in SEQ ID NO: 2, and the The first protein fragment treated with BCEA-Acm modification has a sequence such as SEQ ID NO: 3, and the third protein fragment has a sequence such as SEQ ID NO: 4: thus, Lys27 Diub-BCEA-SH can be efficiently prepared. The inventors were surprised to find that the Lys27 diubiquitin prepared by this method has multiple application values.
[0092] The present invention shows a ...
Embodiment 1
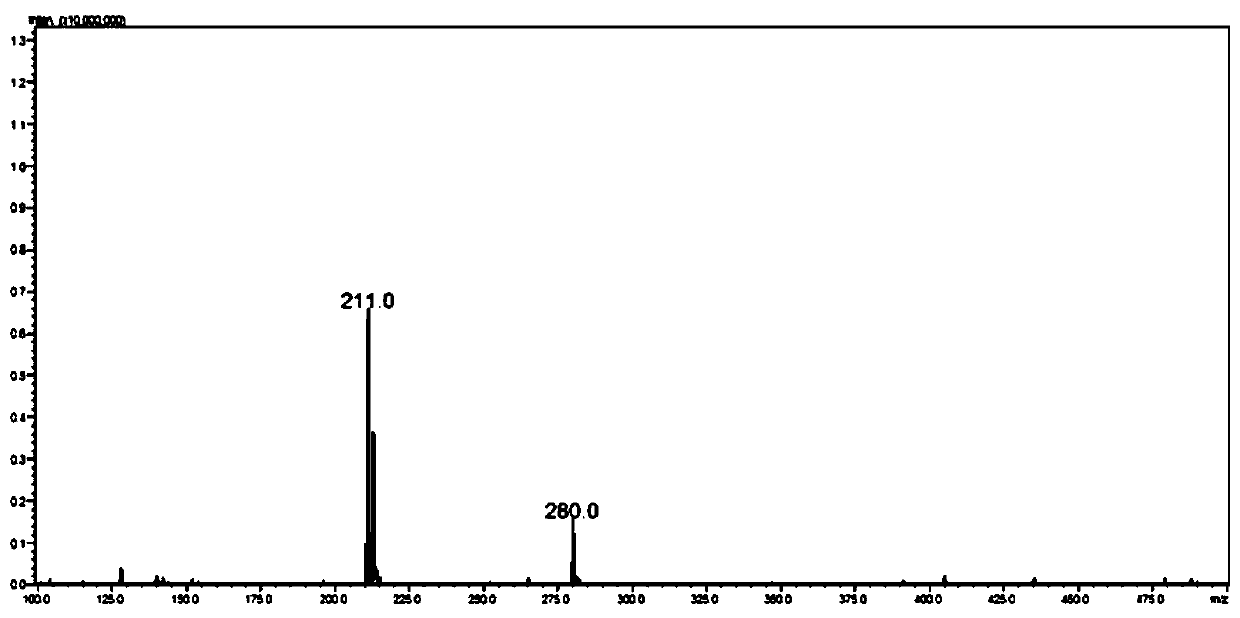
[0095] Embodiment 1: Synthesis of Cl-BCEA-Acm, the compound shown in Formula 1
[0096] The first raw material N-methylolacetamide (0.45g, 5mmol, 1eq) and the second raw material 2-chloro-N-(2-(tritylsulfide)ethyl)ethylamine (1.9g, 5mmol , 1eq) or 2-chloro-N-tert-butoxycarbonyl-N-(2-(tritylsulfide) ethyl)-ethylamine (2.0g, 5mmol, 1eq) mixed, dissolved In 4ml of deionized water, cool in an ice bath for 10 minutes, then slowly add trifluoroacetic acid, trifluoromethanesulfonic acid, and triisopropylsilane in a volume ratio of 92.5:5:2.5 to the ice-cooled mixture. Cleave reagent (24ml), remove the ice room temperature reaction for 4hr. Add an equal volume of 80ml of water and diethyl ether for extraction three times, combine the aqueous phases, freeze-dry after the diethyl ether evaporates to obtain about 1 g of the product, and the yield is 50%. Since by-products do not affect subsequent ligation with protein fragments, no separation is required. The obtained product was dete...
Embodiment 2
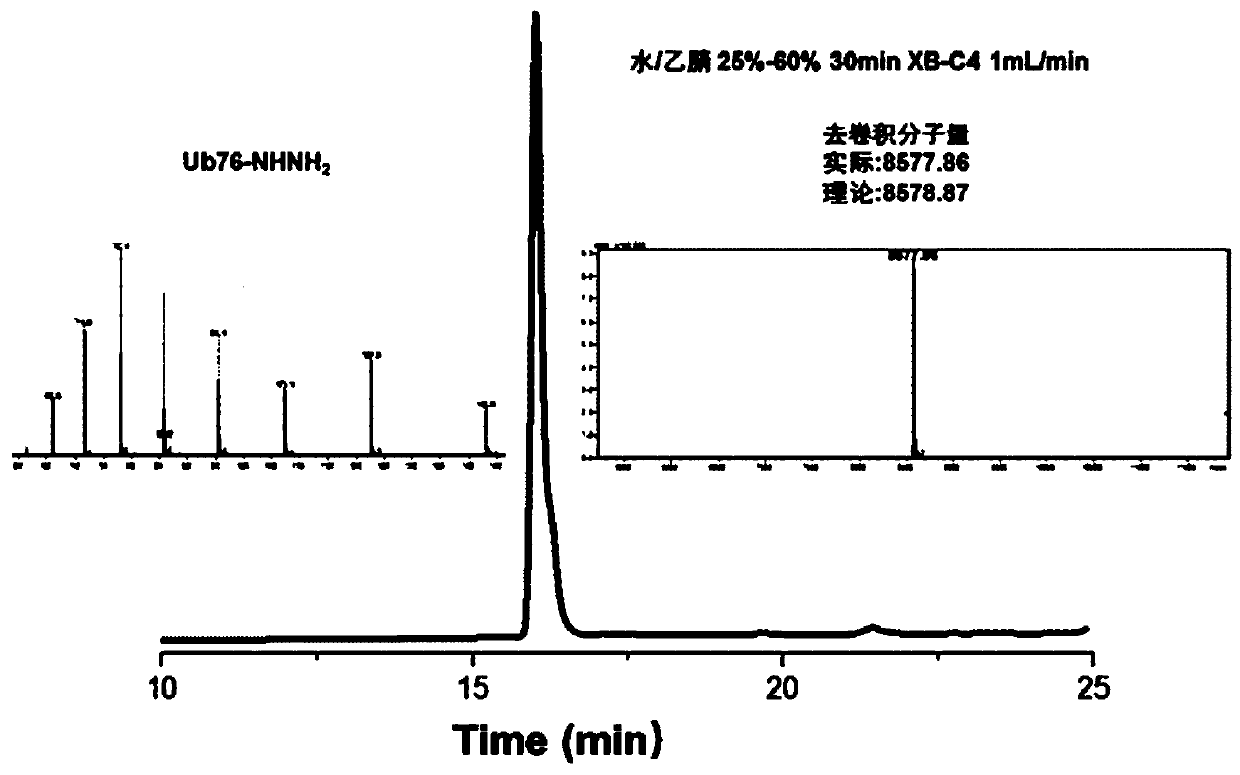
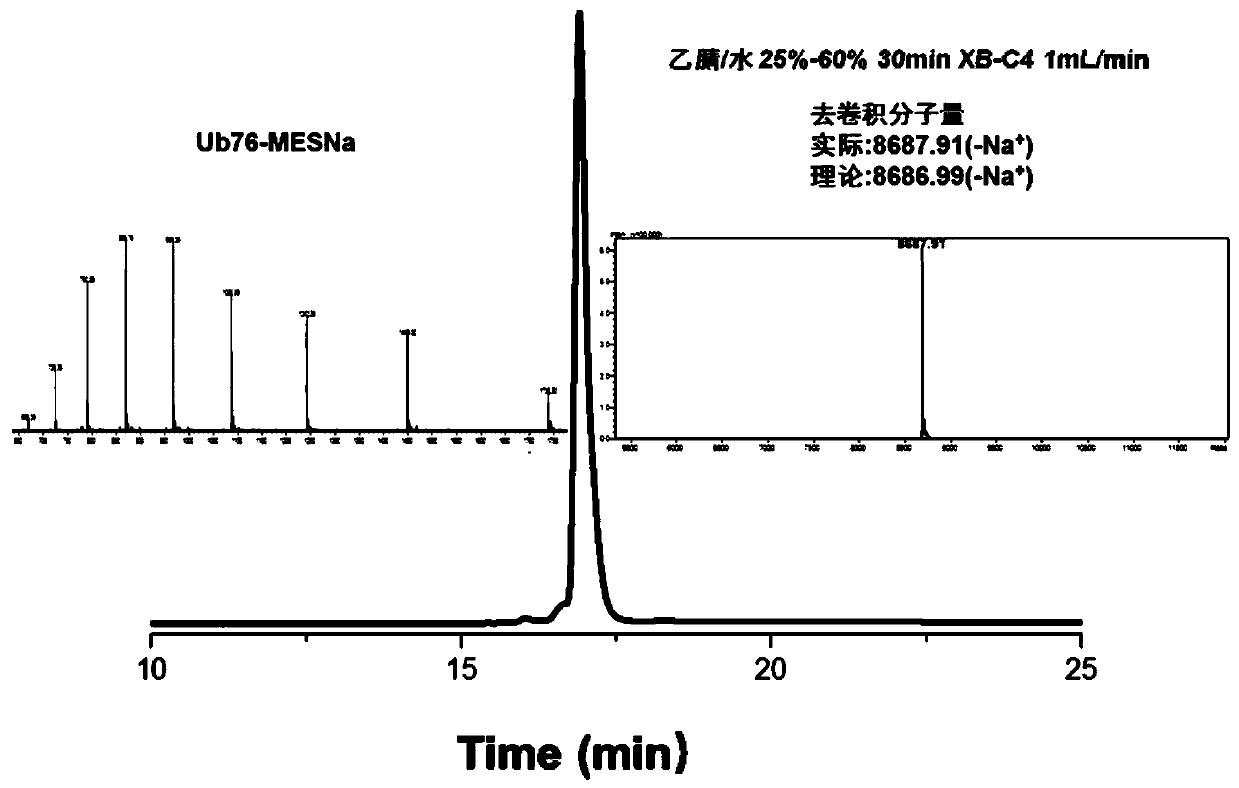
[0097] Example 2: Preparation of Lys27 Diub-BCEA-SH
[0098] 1. Preparation of third protein fragments
[0099] Use Escherichia coli to express Ub77D recombinantly, add 50mM Tris to the bacterial solution, pH 7.50, sonicate, centrifuge, add 1% HClO to the supernatant 4 . Centrifuge at 12000rpm for 1h. Take the supernatant and filter, and dialyze with 3.5K dialysis bag (5-10mM Tris of dialysate) twice. Concentrate Ub77D to a final concentration of 20-30 mg / ml. Add 100mM Tris pH 8.0 solution containing 10% hydrazine hydrate, mix with Ub77D protein solution 1:1 (ub77d final concentration 10-15mg / ml, hydrazine hydrate final concentration 5%), and pre-cool the mixed solution in ice bath for 10min. Add 1uM (final concentration) of YUH1, turn it upside down and put it in an ice bath for reaction, and monitor it in real time with RP-HPLC chromatography. After the conversion of raw materials is complete (30 minutes), use TFA to adjust the pH to 2-3 and a white precipitate appears. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com