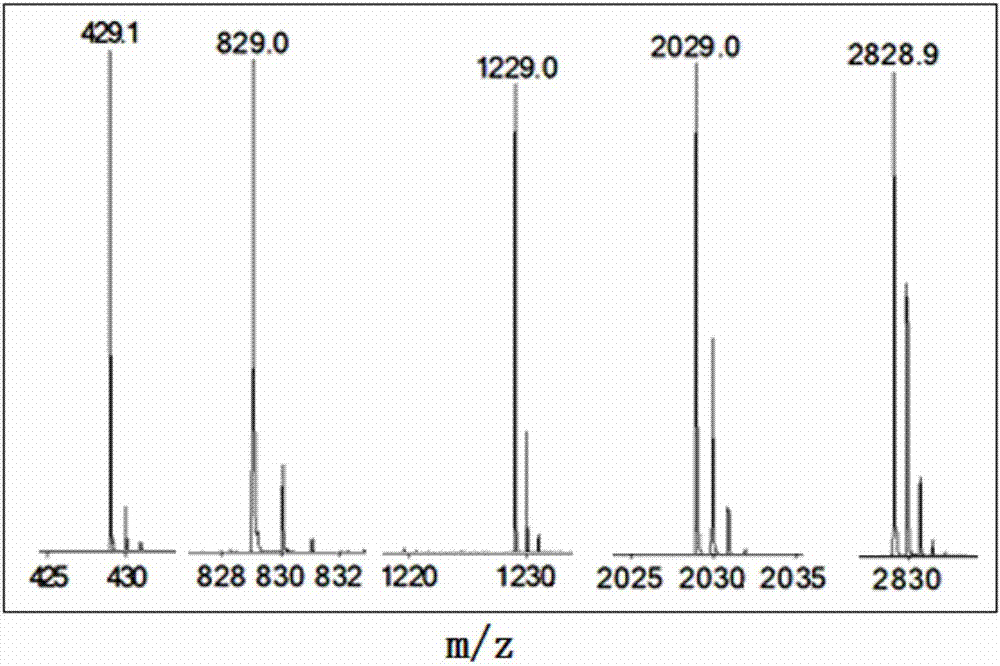
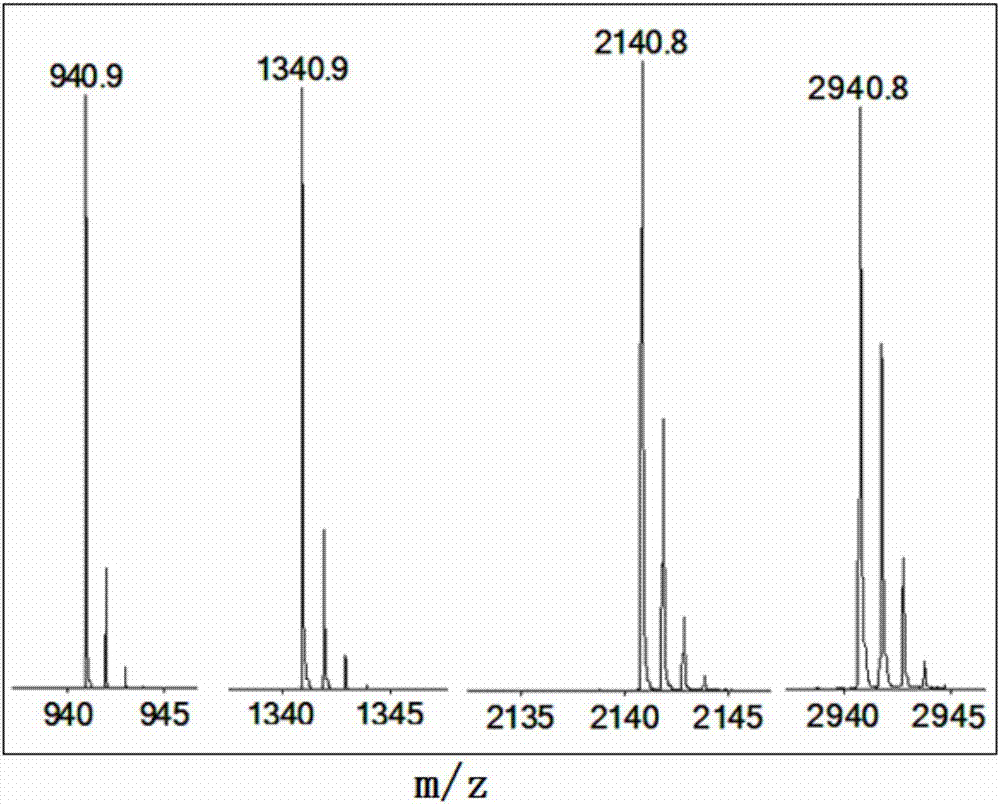
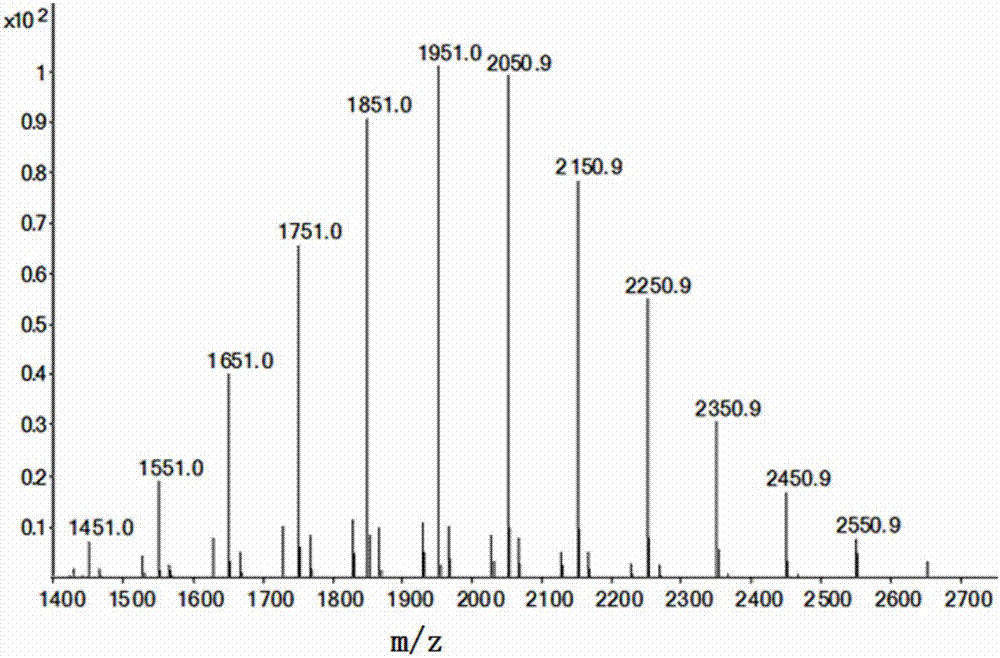
Application of substituted cyclotetraphosphazene compounds
A technology of cyclotetraphosphazene and compound, which is applied in the field of mass spectrometry, can solve the problems of high application concentration, memory effect, unsubstituted cyclotetraphosphazene compound application reports, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Homosubstituted cyclotetraphosphazenes: 2,2,4,4,6,6,8,8-octa(2,2-difluoroethoxy)-2λ 5 ,4λ 5 ,6λ 5 ,8λ 5 - Preparation of cyclotetraphosphazene (SMS-0828):
[0202] Add 104g (0.5mol) of phosphorus pentachloride, 29.4g of ammonium chloride (0.55mol) and 200mL of tetrachloroethane into a 500mL three-necked flask, heat to 147°C and reflux for 7 hours under stirring, to complete the reaction, evaporate the tetrachloride under reduced pressure. Ethane, and the residue was recrystallized from petroleum ether to obtain white crystals, Octachlorocyclotetraphosphazene, 19g, yield: 8.1%, melting point: 123.4-124.3°C;
[0203] Add 5mL of 2,2-difluoroethanol into a 100mL three-neck flask, cool to 0°C in an ice-water bath, add 460mg (20mmol) sodium metal in batches under stirring, remove the ice-water bath, react at room temperature for 30 minutes, then heat and reflux for 2 hours to end the reaction. The excess 2,2-difluoroethanol was distilled off under reduced pressure, and th...
Embodiment 2
[0205] Homosubstituted cyclotetraphosphazenes: 2,2,4,4,6,6,8,8-octa(2,2,3,3-tetrafluoropropoxy)-2λ 5 ,4λ 5 ,6λ 5 ,8λ 5 - Preparation of cyclotetraphosphazene (SMS-1228):
[0206] Add 400mg NaH (60%) and 50mL dry toluene to a 100mL three-necked flask, under nitrogen protection, slowly add dropwise a solution of 1.32g (10mmol) 2,2,3,3-tetrafluoropropanol dissolved in 5mL dry toluene under stirring, After reacting at room temperature for 30 minutes, heat and reflux for 2 hours, cool the reaction solution to 50°C, add dropwise a solution of 464 mg (1 mmol) octachlorocyclotetraphosphazene dissolved in 5 mL of dry toluene, heat and reflux and stir for 5 hours to complete the reaction, and depressurize The toluene was evaporated, and 100 mL of diethyl ether was added to the residue, and the resulting diethyl ether mixture was washed with 2N dilute hydrochloric acid, water, and saturated NaCl solution successively, and then washed with anhydrous MgSO 4 After drying and removing th...
Embodiment 3
[0208] Preparation of heterogeneously substituted cyclotetraphosphazenes (SMS-132-232):
[0209] Add 360mg NaH (60%) and 50mL dry toluene to a 100mL three-necked flask, under nitrogen protection, slowly add 0.58g (4.4mmol) 2,2,3,3-tetrafluoropropanol and 1.02g (4.4mmol) 2 , 2,3,3,4,4,5,5- Octafluoropentanol dissolved in 5mL of dry toluene solution, reacted at room temperature for 30 minutes, heated to reflux for 2 hours, cooled the reaction solution to 50°C, added dropwise 464mg (1mmol ) Octachlorocyclotetraphosphazene dissolved in 5mL of dry toluene solution, heated to reflux and stirred for 5 hours to complete the reaction, evaporated the toluene under reduced pressure, added 100mL ether to the residue, and washed the obtained ether mixture with 2N dilute hydrochloric acid, water, , washed with saturated NaCl solution, anhydrous MgSO 4 After drying, the solvent was spun off, and the solid was purified by column chromatography to obtain a light yellow oily compound (SMS-132-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com