Preparation method for (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal form I
A technology of pyrrolidine acetamide and -4-, which is applied in the field of preparation of -4-hydroxy-2-oxo-1-pyrrolidine acetamide crystal form, can solve the problems of disclosure, preparation method and crystal form research less, No questions about the crystal form of 4-hydroxy-2-oxo-1-pyrrolidineacetamide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Dissolve 60 mg of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide (Chongqing Runze Pharmaceutical Co., Ltd.) in 1 mL of methanol, heat at 50°C, and filter to obtain a supersaturated solution. Seal the solution and place it Cool and crystallize at -19°C for 24 hours, filter and separate, and dry at a temperature of 65°C and a relative humidity of 20% for about 6 hours to obtain crystals.
Embodiment 2
[0026] The (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide crystal obtained in Example 1 was subjected to crystallographic determination.
[0027] Powder Diffraction Determination (XRPD):
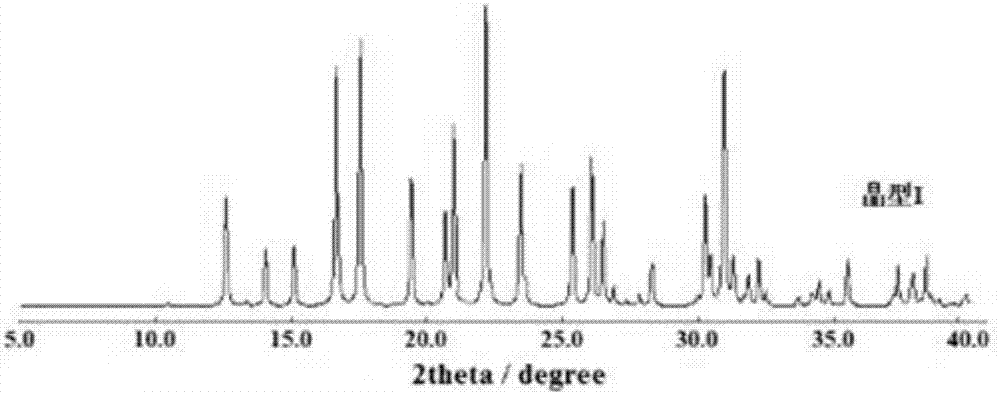
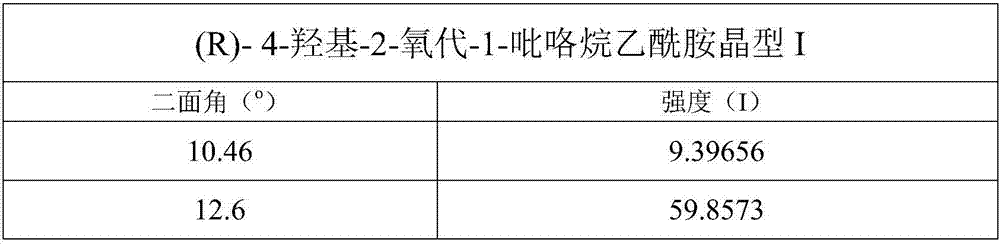
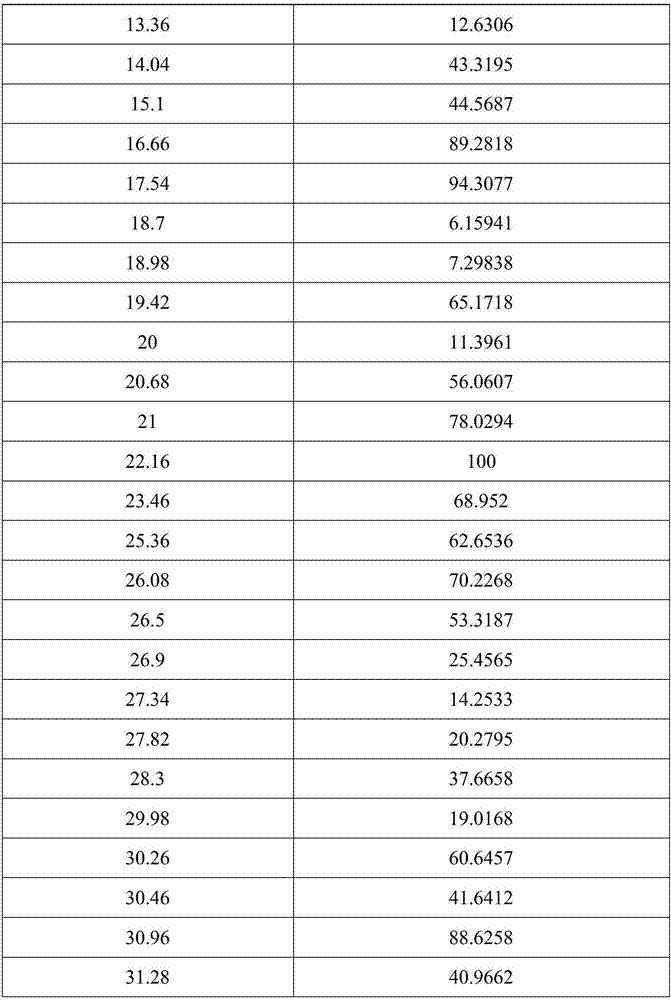
[0028] Test instrument conditions: use Bruker D2PHASER powder diffractometer to test at room temperature, the test conditions are: Cu Ka It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the crystals of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide prepared in Example 1 have diffraction angles 2θ of 12.6±0.2°, 14.04±0.2°, 15.1±0.2°, 16.66 ±0.2°, 17.54±0.2°, 19.42±0.2°, 20.68±0.2°, 21±0.2°, 22.16±0.2°, 23.46±0.2°, 25.36±0.2°, 26.08±0.2°, 26.5±0.2°, 30.26 There are diffraction peaks at ±0.2°, 30.46±0.2°, 30.96±0.2°, 31.28±0.2°. For convenience, this crystal is called "(R)-4-hydroxyl-2-oxo-1-pyrrolidine Acetamide crystal form I", its powder diffraction pattern is shown in ...
Embodiment 3
[0042] Dissolve 100mg of (R)-4-hydroxy-2-oxo-1-pyrrolidineacetamide in 2mL of methanol, heat at 45°C, and filter to obtain a supersaturated solution. Seal the solution and place it at -17°C for cooling Crystallization, separation by filtration, drying at 70°C and a relative humidity of 30% for about 7 hours to obtain colorless sand-like crystals, which were identified as (R)-4-hydroxy-2-oxo- 1-Pyrrolidineacetamide Form I.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com