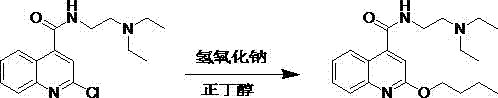
Synthetic method of cinchocaine
Dibucaine and the technology of its synthetic method are applied in the field of medicine and chemical industry, which can solve the problems of unfavorable industrial production, strong alkalinity of sodium butoxide, potential safety hazards, etc., and achieve the effects of improved safety, environmental protection of the synthetic process, and reduction of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The sodium hydroxide of 150g 2-chloro-N-[2-(diethylamino) ethyl]-4-quinoline formamide, 39g is added in the solution of 90g n-butanol, 600g n-hexane, slowly Raise the temperature to reflux for water separation, after the water separation is completed, cool down to room temperature, add deionized water and stir for 1 hour, stand still for 0.5 hours, separate the water phase, add deionized water to the organic phase and stir for 0.5 hours, separate the liquid to remove the water phase, The organic phase was stirred and crystallized at 0-10°C for 8 hours, filtered and dried to obtain 162.5 g of dibucaine fine product, with a molar yield of 96.4% and a liquid phase purity of 99.9%.
Embodiment 2
[0032] The sodium hydroxide of 150g 2-chloro-N-[2-(diethylamino) ethyl]-4-quinoline formamide, 39g is added in the solution of 90g n-butanol, 600g sherwood oil, slowly Raise the temperature to reflux for water separation. After the water separation is completed, cool down to room temperature, add deionized water and stir for 1 hour, and keep stratifying for 0.5 hours. Separate the liquid to remove the water phase. Then add deionized water to the organic phase and stir for 0.5 hours. The aqueous phase was removed by liquid separation, and the organic phase was stirred and crystallized at 0-10°C for 8 hours, filtered and dried to obtain 160.3 g of fine dibucaine, with a molar yield of 95.1% and a liquid phase purity of 99.9%.
Embodiment 3
[0034] 150kg of 2-chloro-N-[2-(diethylamino) ethyl]-4-quinoline carboxamide, 135kg of salt of wormwood are added in the solution of 90kg n-butanol, 600kg of toluene, slowly warming up to Reflux water separation, after water separation is completed, cool down to room temperature, add deionized water and stir for 1 hour, static layering for 2 hours, liquid separation to remove the water phase, add deionized water to the organic phase and stir for 0.5 hours, static layering for 0.5 hours, liquid separation and removal The water phase and the organic phase were stirred and crystallized at 0-10°C for 8 hours, filtered and dried to obtain 158.0 g of fine dibucaine, with a molar yield of 93.8% and a liquid phase purity of 99.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com