Fluorescent probe for specific recognition hydrazine hydrate
A fluorescent probe and species-specific technology, applied in the field of fluorescent probes, can solve the problems of the small number of fluorescent probes for ratiometric detection of hydrazine hydrate and the limited number of fluorescent probes reported, and achieve strong anti-interference ability and low cost , good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of intermediate product 4
[0023] 400 mg of compound 2 and 295 mg of compound 3 were dissolved in 10 mL of toluene, refluxed for 24 hours, filtered with suction, washed with 10 mL of petroleum ether, and dried to obtain 210 mg of off-white powder as compound 4, yield, 54.5%. 1 H NMR (400MHz, DMSO-d 6 )δ11.75(s, 1H), 7.15(s, 1H), 5.21(s, 1H), 3.23(dd, J=11.1, 5.5Hz, 4H), 2.71(dd, J=8.9, 6.1Hz, 4H ), 1.87 (dd, J=11.9, 6.2Hz, 4H). 13 C NMR (100MHz, DMSO-d 6 )δ 167.06, 163.29, 151.47, 146.46, 120.35, 117.78, 105.83, 103.57, 86.28, 49.69, 40.43, 40.22, 40.01, 39.80, 39.59, 27.43, 21.49, 20.62, = 20.58.
Embodiment 2
[0024] Embodiment 2: the synthesis of probe
[0025] Dissolve 51.4 mg of compound 4 in 10 mL of anhydrous dichloromethane, add 85 μL of triethylamine, and bathe in ice-water under argon protection for 30 minutes, then add 25 μL of acetyl chloride, and keep in ice-water bath for 30 minutes. The solvent was spin-dried, and 40 mg of yellow powder was obtained as the chemical probe by column chromatography, and the yield was 66.9%. 1 H NMR (400MHz, CDCl 3)δ6.91(s, 1H), 5.98(s, 1H), 3.27(dd, J=11.8, 6.7Hz, 4H), 2.87(d, J=6.5Hz, 2H), 2.76(s, 2H), 2.39(s, 3H), 2.01–1.91(m, 4H). 13 C NMR (100MHz, CDCl 3 )δ 167.09, 163.27, 159.93, 146.65, 119.49, 118.40, 106.74, 98.52, 77.39, 77.07, 76.75, 49.97, 49.57, 27.71, 21.39, 21.23, 20.47.
Embodiment 3
[0026] Embodiment 3: The present invention: the application of fluorescent probe
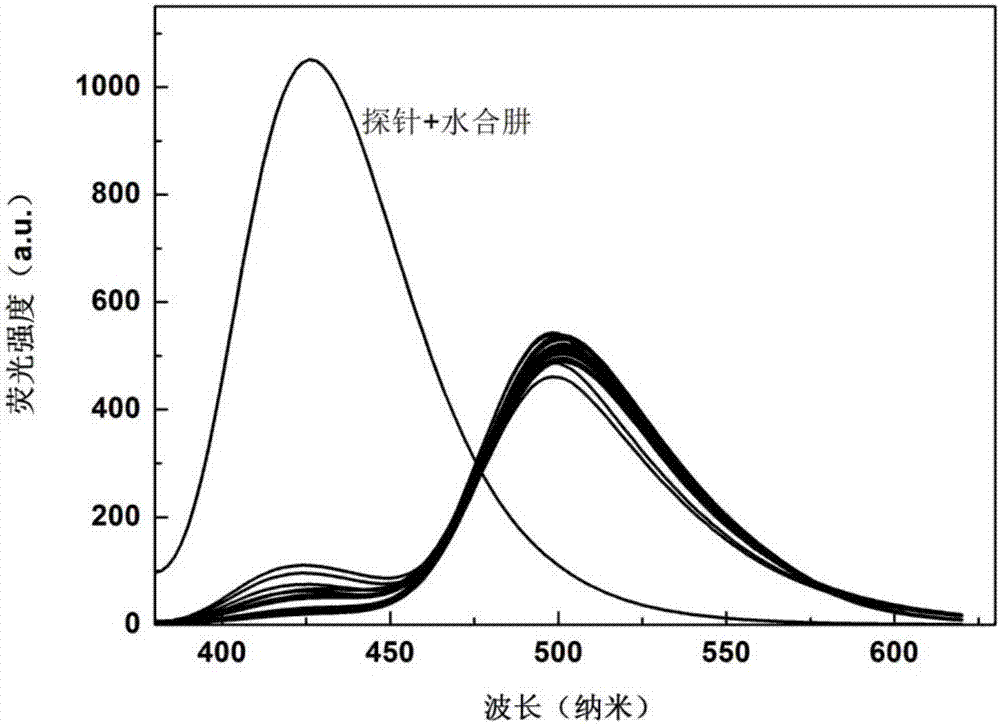
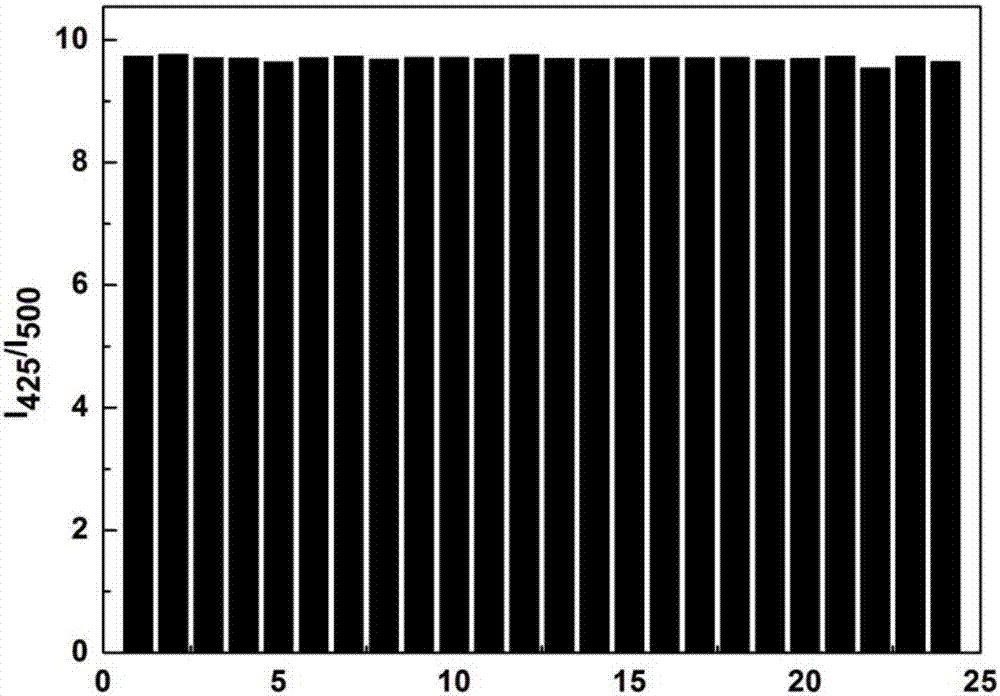
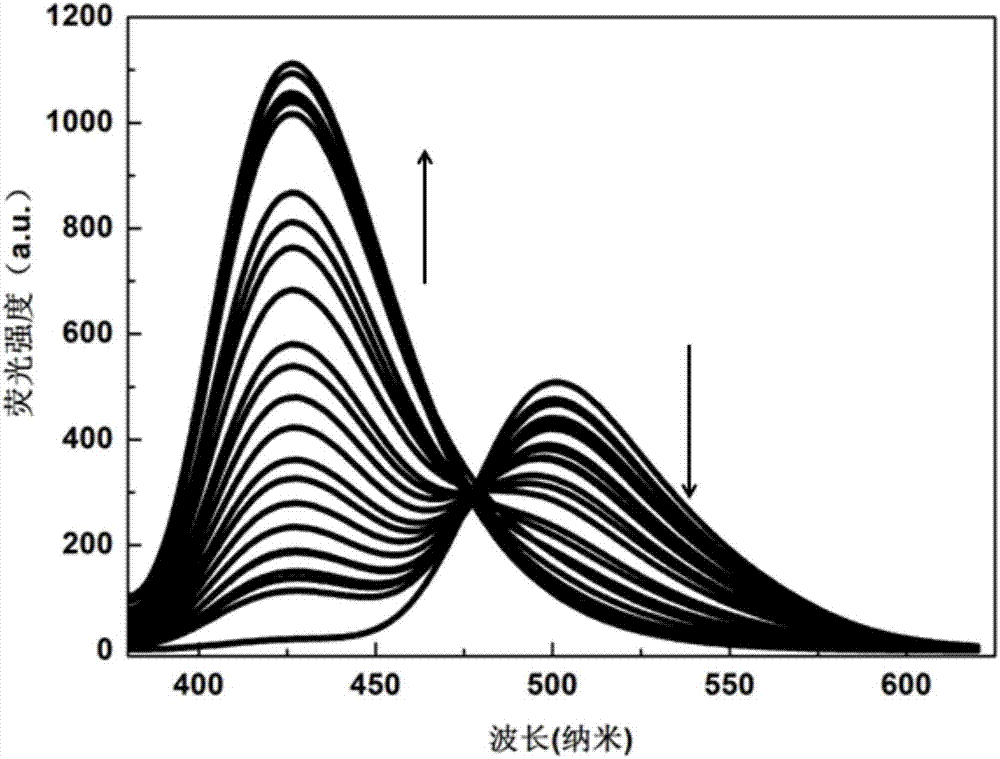
[0027] Dissolve the probe in PBS (10.0mM) buffer solution with pH 7.4, and make 1.0×10 -5 mol / L solution, add other optional substances (Cys, Hcy, GSH, Na + , k + , Mg 2+ , Al 3+ , Zn 2+ , Ca 2+ , N 3 - , F - , CO 3 2- , HPO 4 2- , S 2 o 3 2- , S 2- , SCN - , HCO 3 - , NO 3 - , SO 3 2- , HSO 3 - , NO 2 - , ACO - , HSO 3 - ), the addition of hydrazine hydrate leads to the change of the fluorescence intensity at 500nm and 425nm of the solution, and the fluorescent probe shows high sensitivity and high selectivity for the recognition of hydrazine hydrate. When hydrazine hydrate and interfering substance NaN 3 , AlF 3 , NaF, Na 2 HPO 3 , Na 2 S 2 o 3 , Na 2 S, NaSCN, Na 2 CO 3 , CaCl 2 , KCl, MgCl 2 , NaCl, NaNO 3 , ZnCl 2 , Na 2 SO 4 , Na 2 SO 3 , NaHSO 3 , NaNO 2 , NaACO, Hcy, Cys, GSh, NaHCO 3 When coexisting, the probe showed a strong anti-inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com