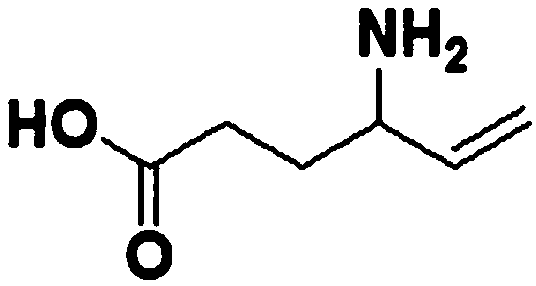
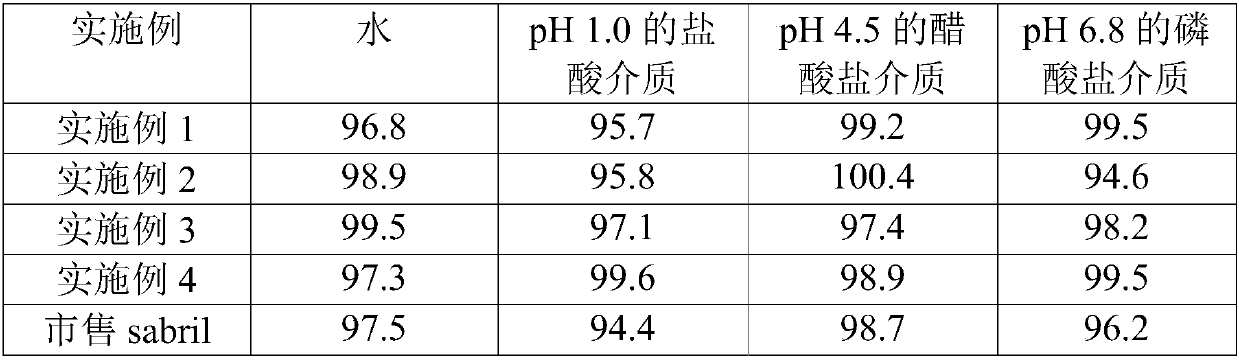
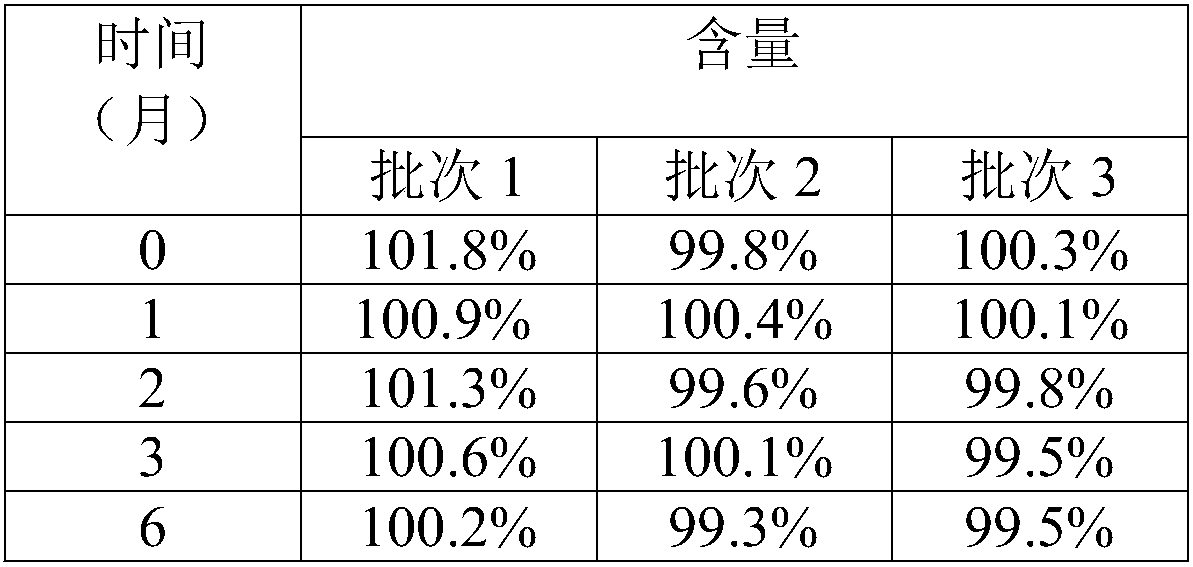
Vigabatrin chewable tablet and preparation method thereof
A technology of vigabatrin and chewable tablets, which is applied in the field of preparation of vigabatrin chewable tablets, can solve the problems of inconvenient taking of vigabatrin oral preparations, and achieve poor drug compliance, good compliance, simple and reliable process The effect of the operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Table 2
[0049] Element
Dosage for 1000 tablets (g)
mass percentage
Vigabatrin 160101
250
60%
110
26.4%
Aspartose
25
6%
4
0.9%
iron dioxide
3
0.7%
12
2.8%
10
2.4%
3
0.7%
[0050] Preparation Process
[0051] (1) Pass the auxiliary material through an 80-mesh sieve, and set aside;
[0052] (2) Weigh hypromellose, add 95% ethanol solution, stir and dissolve, and prepare 5% hypromellose solution as a binder.
[0053] (3) According to the mass percentage content of each component in the tablet in Table 2, take vigabatrin, mannitol, aspartose, menthol, iron dioxide, sodium starch glycolate and place in In the wet mixing granulator, mix for a few minutes until uniform, then add the prepared binder hydroxypropyl cellulose solution, cut and stir to make a soft material.
[0...
Embodiment 2
[0058] table 3
[0059] Element
Dosage for 1000 tablets (g)
weight percentage
Vigabatrin 160102
250
58.7%
70
16.4%
30
7.0%
25
5.9%
Lemon Spice
6
1.4%
iron dioxide
3
0.7%
Low-substituted hydroxypropyl cellulose
16
3.8%
20
4.7%
silica
6
1.4%
[0060] Preparation Process
[0061] (1) Pass the auxiliary material through an 80-mesh sieve, and set aside;
[0062] (2) Weigh polyvinylpyrrolidone, add 95% ethanol solution, stir to dissolve, and prepare 8% adhesive solution.
[0063] (3) According to the mass percentage content of each component in the tablet in Table 3, weigh vigabatrin raw materials, xylitol, starch, sodium saccharin, lemon flavor, low-substituted hydroxypropyl cellulose and dioxide The iron is placed in a wet mixing granulator, mixed for a few minutes until uniform, then the p...
Embodiment 3
[0068] Table 4
[0069] Element
Dosage for 1000 tablets (g)
weight percentage
Vigabatrin 160103
100
40%
100
40%
Cyclamate
15
6%
Taro Spice
4
1.6%
iron dioxide
2
0.8%
14
5.6%
Crospovidone
10
4%
5
2%
[0070] Preparation Process
[0071] (1) Pass the auxiliary material through an 80-mesh sieve, and set aside;
[0072] (2) Weigh hydroxypropyl cellulose, add 95% ethanol solution, stir and dissolve, and prepare 5% binder solution
[0073] (3) According to the mass percentage content of each component in the tablet in Table 4, weigh vigabatrin raw material, microcrystalline cellulose, cyclamate, taro flavor, iron dioxide and place it in wet mixing granulation In the machine, mix for a few minutes until uniform, then add an appropriate amount of 4% hypromellose solution binder, cut and sti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com