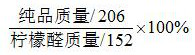A kind of synthetic method of α-isomethyl ionone
The technology of isomethyl ionone and methyl ionone is applied in the field of synthesizing α-isomethyl ionone fragrance and can solve the problem of low selectivity of α-isomethyl ionone and content of α-isomethyl ionone low production cost, few processing steps, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
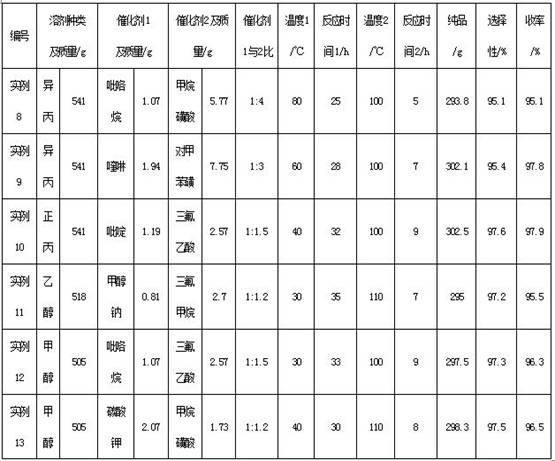
[0030] Embodiment 1 A kind of synthetic method of α-isomethyl ionone
[0031] Include the following steps:
[0032] (1) Add 228.0g of citral, 432.5g of methyl ethyl ketone and 648.5g of solvent methanol into the autoclave for mixing under the protection of nitrogen, add 1.50g of catalyst 1 (pyrrolidine) into the autoclave, replace with nitrogen, and control the reaction temperature at 50°C, heat preservation for 25 hours to detect 0.45% citral residue, and obtain pseudo-methylionone;
[0033] (2) Add 6.32g of catalyst 2 (trifluoromethanesulfonic acid) under the protection of nitrogen, stir evenly and raise the temperature to 110°C, continue the reaction, keep it warm for 5h, and detect that the content of pseudomethyl ionone is 0.3%. After the reaction is completed, add Sodium carbonate was neutralized, solvent 647.5g was reclaimed, butanone 321g was reclaimed to obtain the crude product, and then 304.7g of α-isomethylionone pure product was rectified at an absolute pressure ...
Embodiment 2
[0034] Embodiment 2 A kind of synthetic method of α-isomethyl ionone
[0035] Include the following steps:
[0036] (1) Add citral 228.0g, butanone 216.2g and solvent polyethylene glycol 200:600.6g into the autoclave for mixing under nitrogen protection, add catalyst 1 (potassium carbonate) 0.21g into the autoclave, and replace with nitrogen , control the reaction temperature at 40°C, keep the temperature for 40 hours to detect 0.48% of the residual citral, and obtain pseudo-methylionone;
[0037] (2) Add 0.78g of catalyst 2 (p-toluenesulfonic acid) under the protection of nitrogen and stir evenly, then raise the temperature to 130°C, continue the reaction, keep it warm for 10h, and detect that the content of pseudomethylionone is 0.35%, and the reaction is over , add sodium carbonate to neutralize; reclaim 600g of solvent, reclaim butanone 103.7g, obtain crude product, then rectify 302.1g of α-isomethyl ionone pure product at absolute pressure 100pa, in terms of citral, the ...
Embodiment 3
[0038] Embodiment 3 A kind of synthetic method of α-isomethyl ionone
[0039] Include the following steps:
[0040] (1) Add 228.2g of citral, 648.1g of methyl ethyl ketone and 810.4g of solvent water into the autoclave for mixing under the protection of nitrogen, add 4.05g of catalyst 1 (sodium methoxide) into the autoclave, replace with nitrogen, and control the reaction temperature at -10°C, heat preservation for 40 hours to detect 0.42% citral residue, and obtain pseudo-methylionone;
[0041] (2) Add 17.10 g of catalyst 2 (trifluoroacetic acid) under the protection of nitrogen and stir evenly, then raise the temperature to 80°C, continue the reaction, keep it warm for 8 hours, and detect that the content of pseudomethyl ionone is 0.52%. After the reaction is completed, add carbonic acid Sodium neutralization; recovery of solvent 809.7g, recovery of methyl ethyl ketone 535.74g, to obtain the crude product, and then rectifying 295.6g of pure α-isomethyl ionone at an absolute...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com