A kind of preservation method and special protective agent of Salmonella choleraesuis vaccine strain
A technology of Salmonella cholera and Salmonella, which is applied in the field of preparation of auxiliary preparations for vaccine products, can solve the problems of increased intracellular solute concentration, increased solute damage, excessive dehydration of cells, etc., and achieves good storage stability, easy operation, and high survival rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1: Screening of protective agents for vaccine bacteria under liquid nitrogen ultra-low temperature freezing conditions
[0019] 1.1 Fermentation and concentration of bacterial liquid
[0020] Take the bacterium liquid of the Salmonella choleraesuis vaccinia strain that has been correctly identified by enzyme digestion (the S. choleraesuis vaccinia strain is C500 / pGS / 2SS) and inoculate it in 5ml of LB liquid LB medium according to the volume ratio of 1:100 , placed on an air bath constant temperature shaker at 37°C at 200r / min for 14h, then inoculated the cultured bacterial solution into 350ml LB liquid LB medium at a ratio of 1:100 by volume, at 37°C, 200r / min Shake culture for 15 hours, centrifuge at 4000r / min for 10 minutes, discard the supernatant, and use sterile phosphate buffered saline (abbreviated as PBS, i.e. phosphate buffered saline, pH7.2, concentration 0.01M: weigh disodium hydrogen phosphate (Na 2 HPO 4 12H 2 (O) 2.90g, potassium dihydrogen phos...
Embodiment 2
[0050] Example 2: Compatibility optimization of protective agent for vaccine bacteria C500 / pGS / 2SS cryopreserved in liquid nitrogen
[0051] 2.1 Orthogonal design
[0052] Table 6 L9(3 4 ) Orthogonal design table
[0053]
[0054] Table 7 Liquid nitrogen ultra-low temperature freezing multi-factor optimization test arrangement
[0055]
[0056] Mix the adjusted bacterial solution and the above protective agent formula in equal volumes according to the volume ratio of 1:1 to make the final concentration as shown in Table 7, and then distribute them in cryopreservation tubes. Each group has three replicates and placed in - Place it in a refrigerator at 80°C for 12 hours, then put it into liquid nitrogen for storage for a week, then immediately place the liquid nitrogen-preserved Salmonella choleraesuis vaccine strain in a 37°C water bath for 30 minutes, and then detect the survival rate of the bacteria to screen out the protective bacteria. The most effective protectant...
Embodiment 3
[0065] Embodiment 3: Detection of storage stability of Salmonella choleraesuis vaccine strain C500 / pGS / 2SS
[0066] 3.1 Experimental design
[0067] Under the best protective agent formula screened out, the bacterial liquid of Salmonella choleraesuis vaccine strain C500 / pGS / 2SS with different concentrations was tested, and its storage stability was tested for 1 week and 4 weeks, and in At the end of the experiment, the plasmid stability was checked.
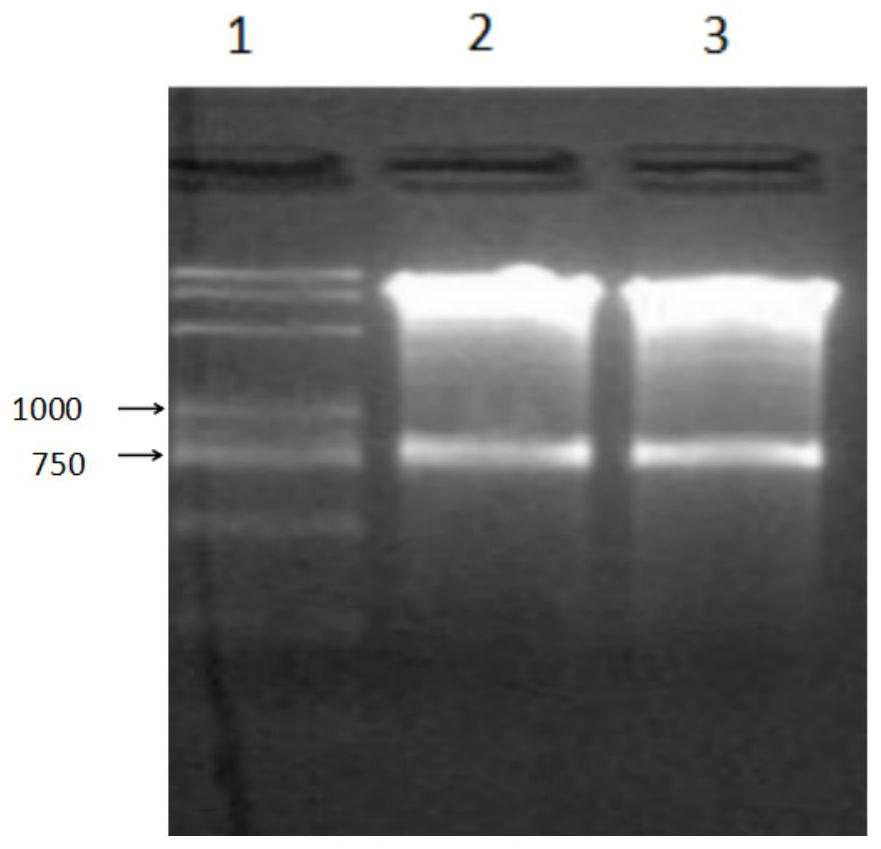
[0068] 3.2 Plasmid mini-extraction and enzyme digestion identification
[0069] On the ultra-clean workbench, use a pipette to take 1ml of the bacterial solution (vaccine bacteria C500 / pGS / 2SS), and follow the instructions of the plasmid mini-extraction kit produced by Tiangen Biochemical (Beijing) Technology Co., Ltd. to extract the plasmid. The specific operation steps are as follows:
[0070] (1) Take 1.5 mL of bacterial liquid in a 2 mL clean centrifuge tube, centrifuge at 12,000 rpm for 1 min, discard the supernatant, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com