Cefcapene pivoxil hydrochloride synthesis method
A cefcapene hydrochloride and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems of unsuitability for large-scale production, complicated operation, low total yield, etc., and achieve saving of drying process, simplified operation steps, and short process cycle Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
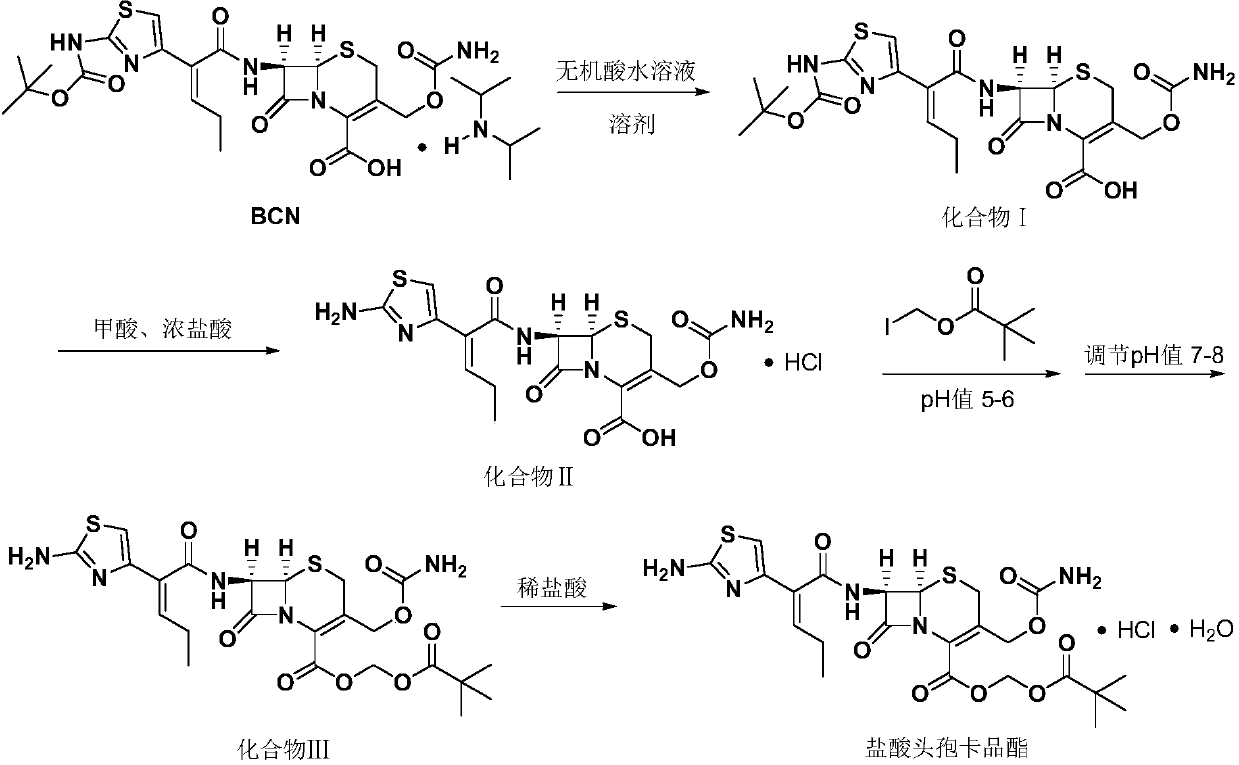
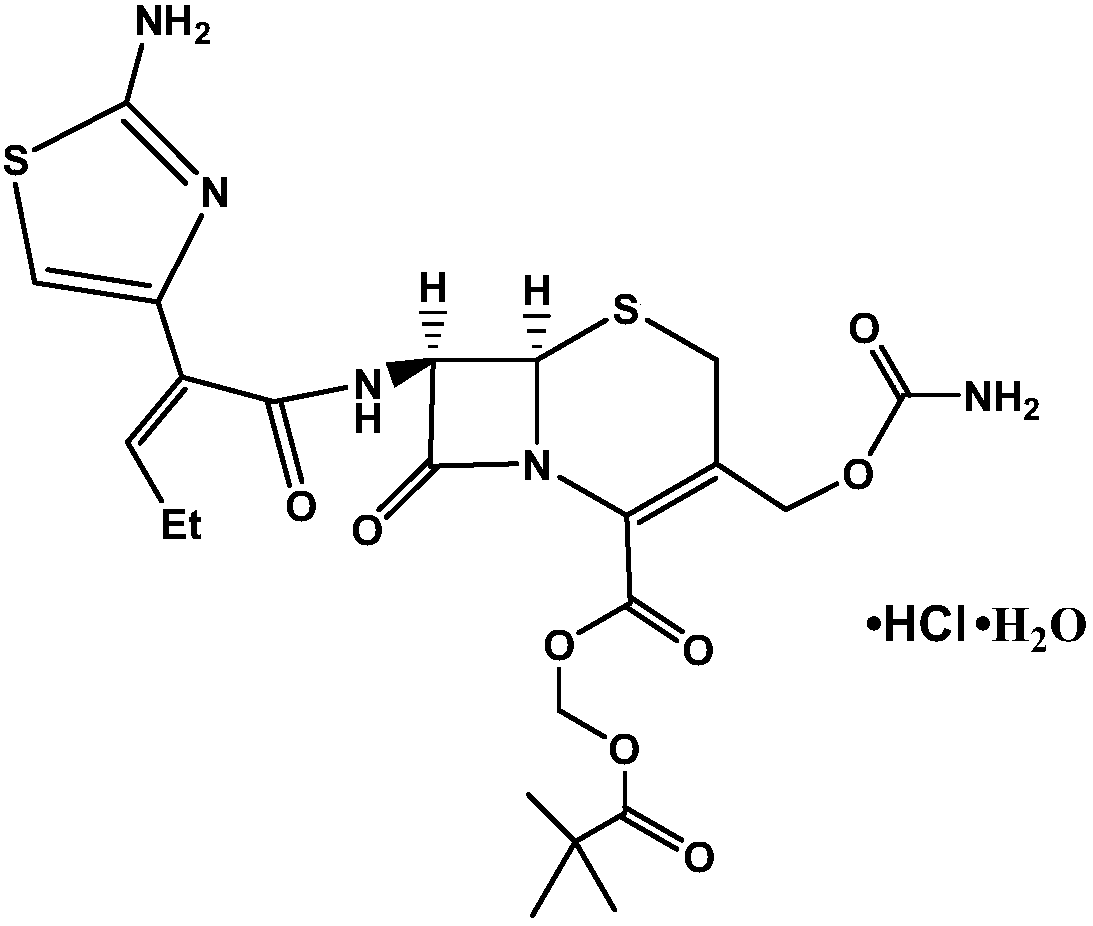
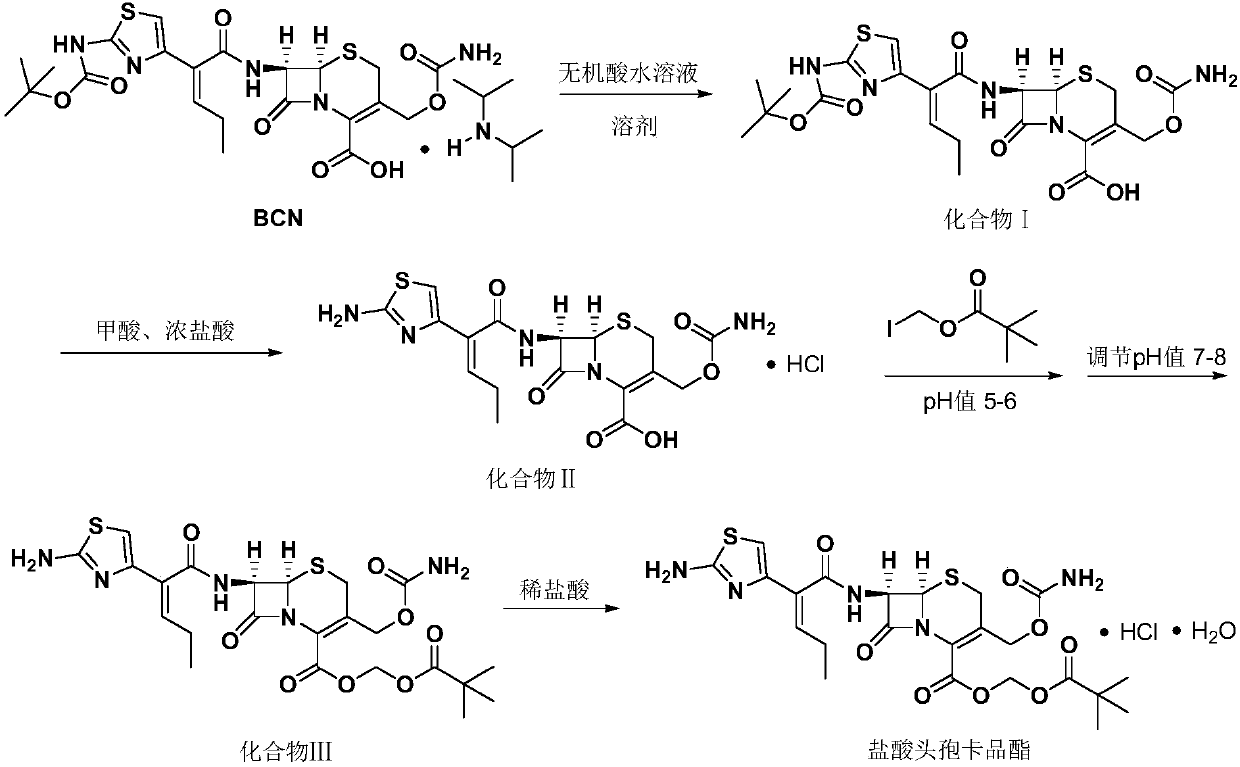
[0033] In a 250ml four-necked flask, add 12.1g (0.0185mol) of BCN solids, 50ml of dichloromethane, add 9.06g (0.00925mol) of 10wt% dilute sulfuric acid to adjust the pH (5.0) to dissolve, stir at room temperature for 10 minutes, and let stand layer, the organic layer was added with 5g of anhydrous sodium sulfate and dried for 8 hours, the water layer was discarded, and after removing the anhydrous sodium sulfate by filtration, 2.7g (0.037mol) of formic acid was added, 18.76g (0.185mol) of 36wt% concentrated hydrochloric acid, and the temperature was controlled 0-5 ℃, insulated and stirred for 7 hours, after the completion of the reaction, add 30ml of water, separate layers, add 4.47 grams (0.0185mol) of iodomethyl pivalate dropwise to the organic layer and maintain pH=6.0 with 7wt% sodium bicarbonate aqueous solution, in React at 25°C for 4 hours. After the reaction is over, add 10ml of ethanol and 30ml of water to wash and separate the layers, discard the water layer, then add...
Embodiment 2
[0035] In a 250ml four-neck flask, add 12.1g (0.0185mol) of BCN solids and 50ml of dichloromethane, stir at room temperature, add 9.07g of 10wt% dilute sulfuric acid to adjust the pH (5.01) to dissolve, let stand and separate, add 5g to the organic layer Anhydrous sodium sulfate was left to stand for 8 hours, the water layer was discarded, and after removing anhydrous sodium sulfate by filtration, 2.7 g (0.037 mol) of formic acid and concentrated hydrochloric acid (22.51 g, 0.222 mol) were added, and the temperature was controlled at 0-5°C, and kept stirring for 7 Hours, add 30ml of water, separate layers, add 4.47 grams (0.0185mol) of iodomethyl pivalate (0.0185mol) and 7wt% sodium bicarbonate aqueous solution to the organic layer dropwise, maintain pH = 6, react at 25 ° C for 5h, after the reaction, wash with ethanol 10ml Wash and separate with 30ml of water, then add 10ml of ethanol, adjust the pH to neutral with 7wt% sodium bicarbonate aqueous solution (23g), separate layer...
Embodiment 3
[0037]In a 250ml four-neck flask, add 12.1g (0.0185mol) of BCN solids and 50ml of dichloromethane, stir at room temperature, add 6.75g (0.0185mol) of 10wt% dilute hydrochloric acid to adjust the pH (5.02) to dissolve and clear, let stand and separate, Add 5 g of magnesium sulfate to the organic layer to dry, filter to remove anhydrous magnesium sulfate, add 2.7 g (0.037 mol) of formic acid, 24.6 g (0.246 mol) of concentrated hydrochloric acid, control the temperature at 0-5 ° C, keep stirring for 7 hours, add 30 ml of water, divide layer, the organic layer was added dropwise with iodomethyl pivalate 4.47 grams (0.0185mol) and 7wt% sodium bicarbonate aqueous solution (40 milliliters), to maintain pH = 5, 30 ° C reaction 3h, after the reaction, with ethanol 10ml and water 30ml Wash and separate the layers, then add 10ml of ethanol, adjust the pH to neutral with 7wt% sodium bicarbonate aqueous solution (26g), separate the layers, concentrate the organic layer under reduced pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com