RPA primer and probe for detecting Listeria monocytogenes and detection method
A technology for mononucleosis and Listeria, which is applied in the field of molecular biology detection, can solve the problems of long detection time, long detection cycle, non-specific amplification, etc., and achieve the effect of being easy to promote and use, and exempting investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Selection of target genes and design and screening of RPA primers and probes
[0039] Use bioinformatics knowledge and related analysis software to analyze common virulence genes of Listeria monocytogenes, such as hly, inl, actA, etc., and compare these genes of Listeria monocytogenes with other microorganisms For comparison analysis, actA was used as the target gene to be selected, and sequence fragments with higher specificity were selected. According to the RPA design requirements for primers and probes, design specific primers and probes, and then compare the sequences of the primers and probes with the species with high homology of Listeria monocytogenes to select the specificity High primers and probes. Then compare and analyze the primers and probes to be selected in the same process, and select a combination with high specificity and high amplification efficiency. The sequences of the primers and probes obtained by screening are shown respectively (SEQ ID No.1...
Embodiment 2
[0045] Establishment of RPA method for Listeria monocytogenes
[0046]Inoculate Listeria monocytogenes CICC 21632 into the nutrient broth, place it on a shaker, and culture overnight according to the optimum growth temperature of each bacterium, draw 1mL of the culture solution and drop it into a 1.5mL centrifuge tube, 12 000r / Centrifuge for 2 min, discard the supernatant, add 500 μL sterile saline, suspend and mix well, centrifuge at 12 000 r / min for 1 min, discard the supernatant, add sterile saline repeatedly, centrifuge again, and discard the supernatant. Add 50 μL of normal saline, put in a water bath at 100°C for 10-15min, centrifuge at 12000r / min for 1min after cooling down to room temperature, and store the supernatant at -20°C for later use.
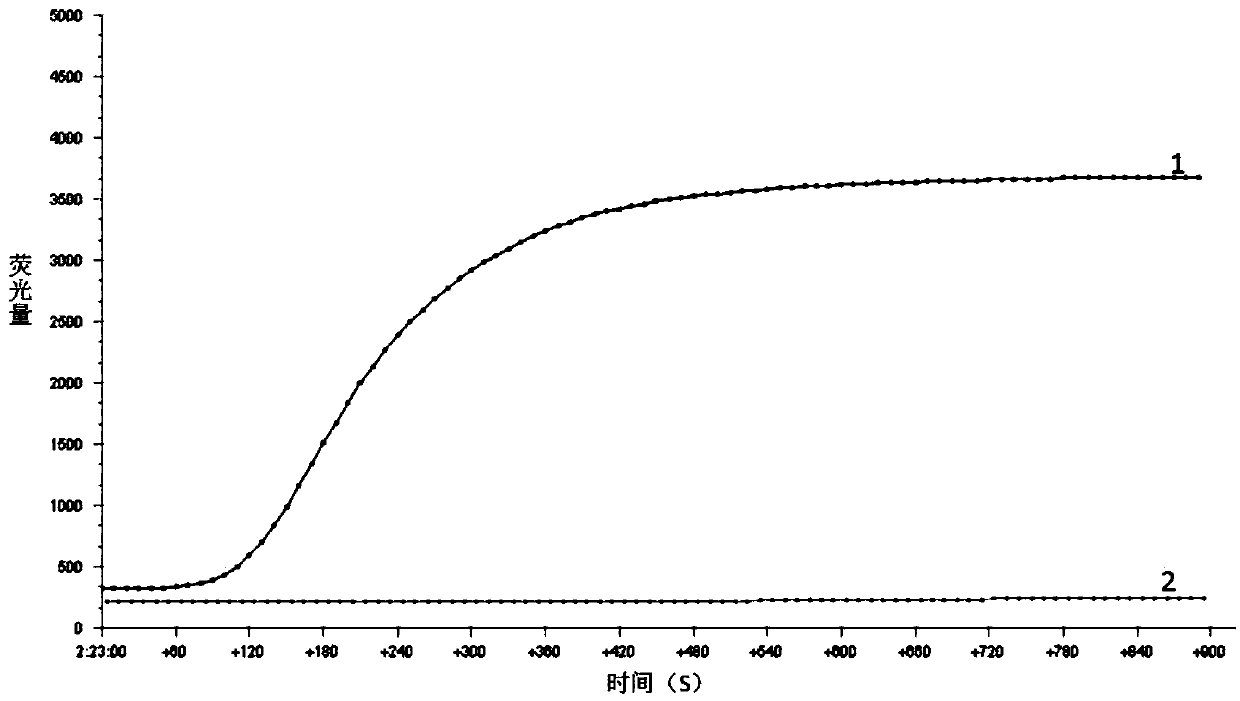
[0047] Using the DNA of Listeria monocytogenes CICC 21632 as a template, set a blank control at the same time, utilize the RPA primers and probes in Example 1 to carry out RPA amplification, and use a fluorescence collection de...
Embodiment 3
[0051] Specific test analysis of the established RPA method
[0052] The standard strains used in this example were all purchased by Guangzhou Huankai Microbiology Co., Ltd., and the specific information is shown in Table 1.
[0053] Table 1 Strains used for RPA specificity analysis
[0054]
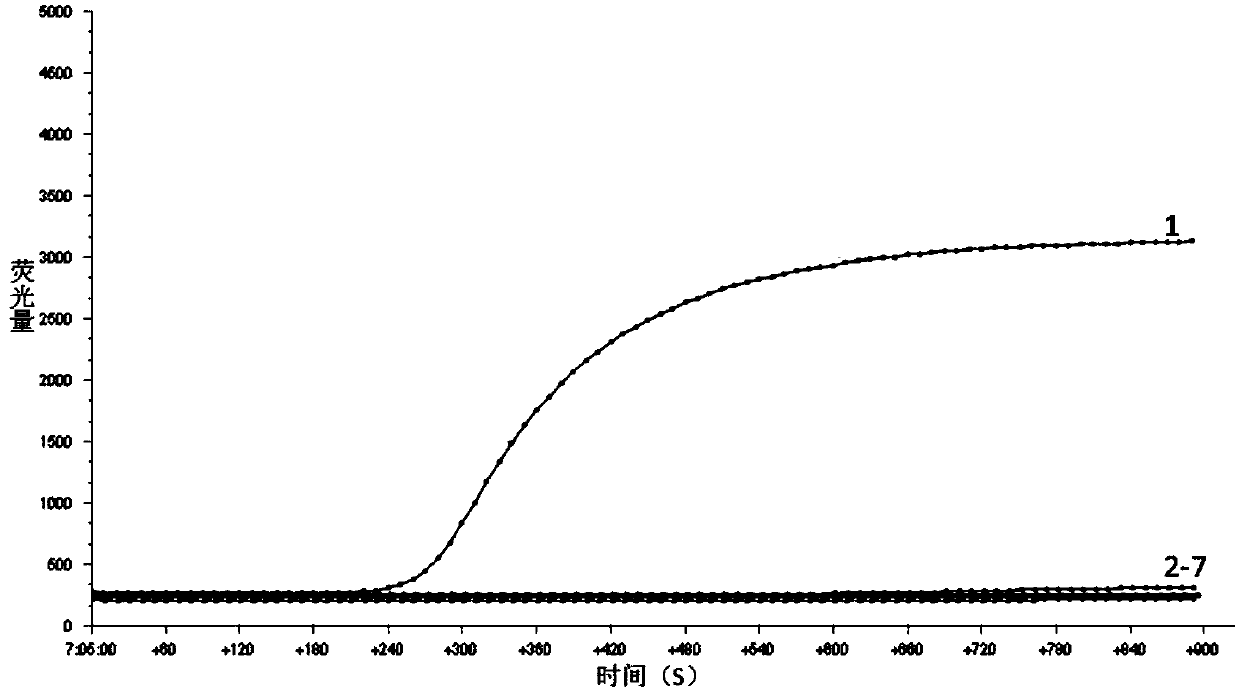
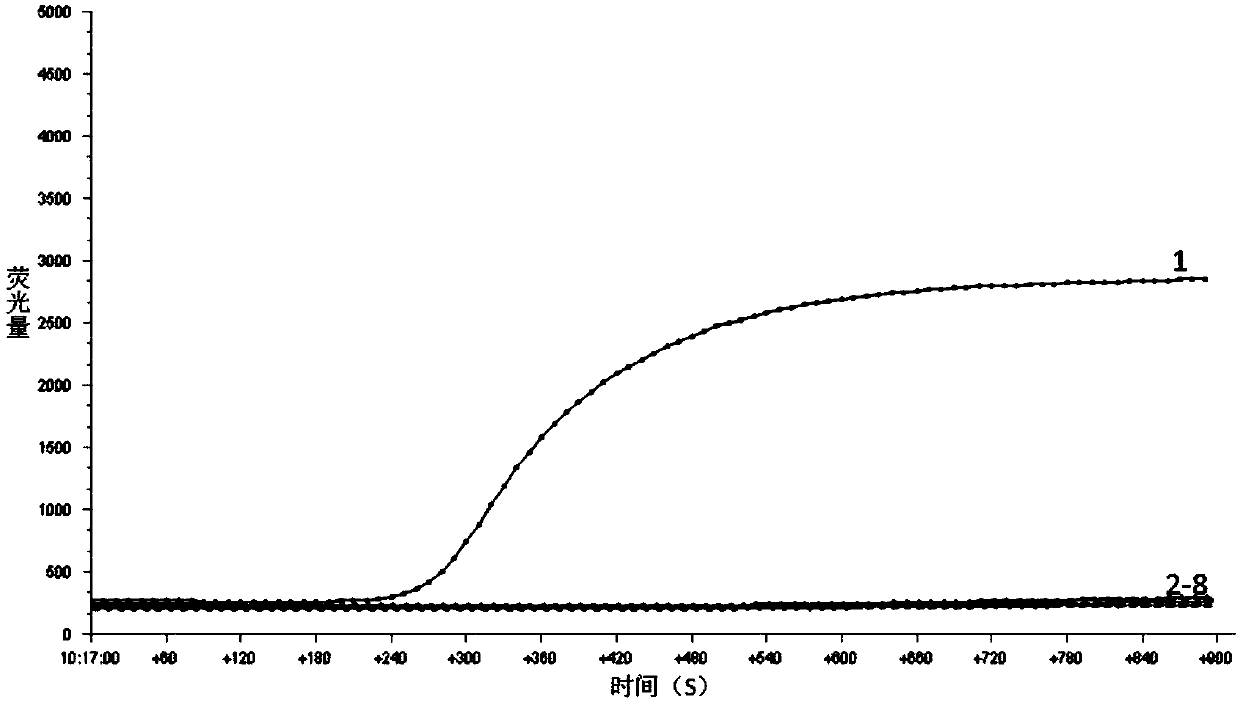
[0055] The strain DNA in Table 1 was used as the RPA reaction template, and the RPA primers and probes in Example 1 were used for RPA amplification.
[0056] The RPA amplification system is as follows: add 29.5uL of rehydration buffer, 11.2uL of deionized water, 2.1uL of upstream and downstream primers, 0.6uL of probe, and 2uL of template to the RPA reaction tube containing lyophilized enzyme powder, and finally add Magnesium acetate solution 2.5uL.
[0057] RPA reaction conditions: Mix the above RPA reaction system well, and amplify at 39°C for 15 minutes.
[0058] Amplification results such as figure 2 and image 3 As shown, it can be known from the results that only the ampli...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com