Preparation method of pentafluorophenol
A technology of pentafluorophenol and pentafluorobenzonitrile, which is applied in the field of preparation of pentafluorophenol, and can solve the problems of low safety, cumbersome operation steps, high cost of raw materials and processes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
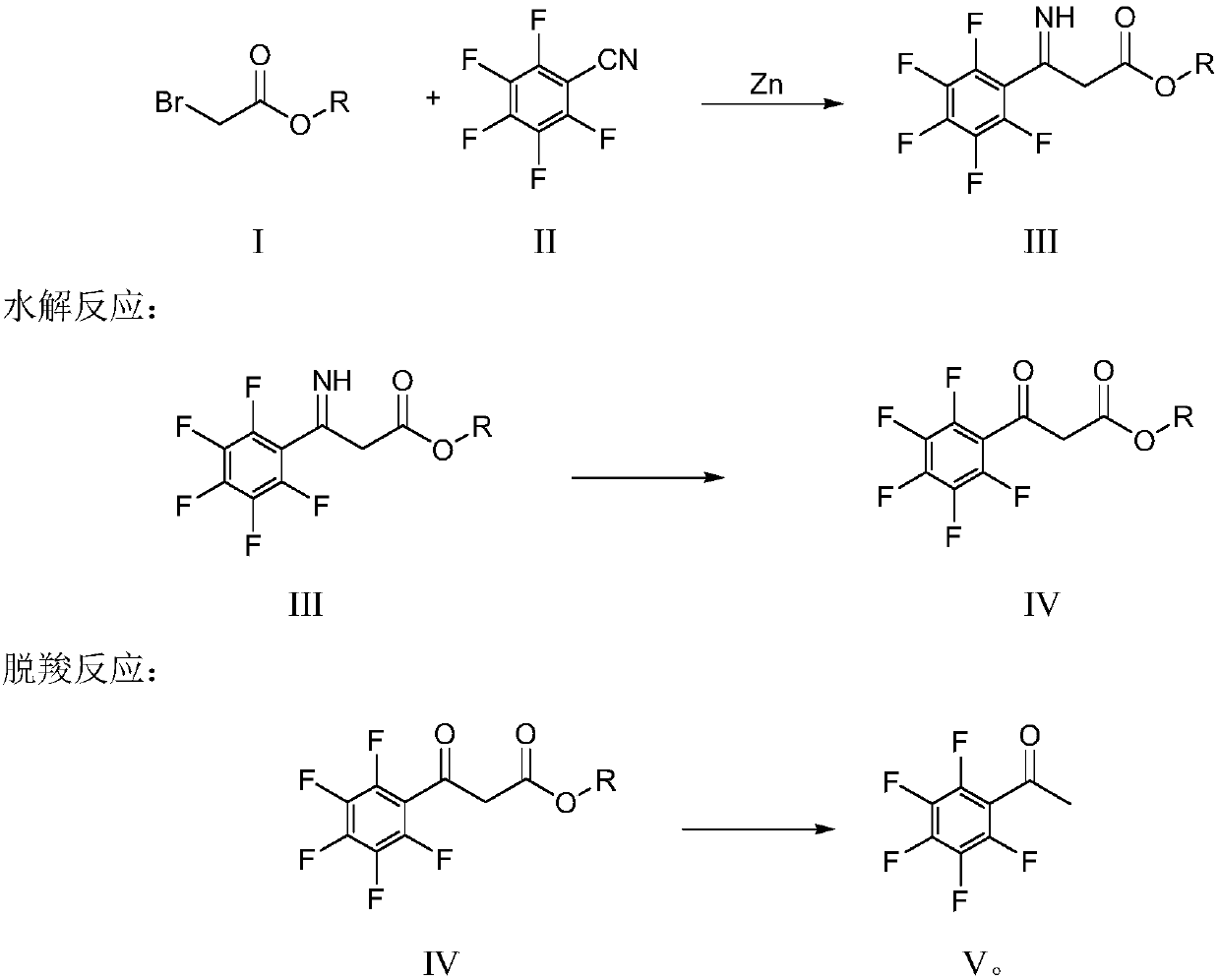
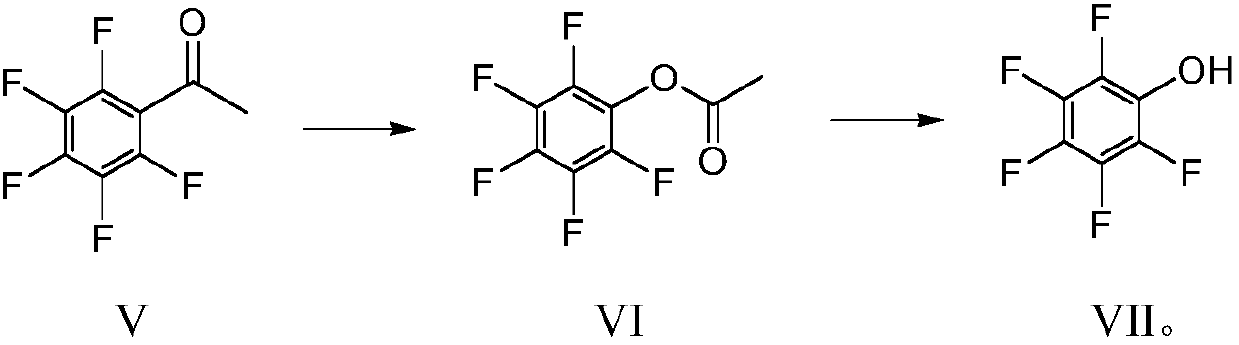
[0039] The present invention provides a kind of preparation method of pentafluorophenol (compound of formula VII), described preparation method can comprise: pentafluorobenzonitrile (compound of formula II) and bromoacetate (compound of formula I) in the condition that zinc powder exists Carry out the coupling reaction under acidic conditions, then hydrolyze and decarboxylate under acidic conditions to prepare pentafluoroacetophenone (compound of formula V), and the reaction equation is as follows:
[0040] coupling reaction:
[0041]
[0042] In the preparation method provided by the present invention, the coupling reaction can be carried out in the presence of a solvent, and those skilled in the art can select the appropriate type and amount of solvent so that the substance can be fully dispersed in the reaction system. The solvent can be an organic solvent, more specifically an ether solvent, and the ether solvent can be, for example, diethyl ether, methyl tert-butyl eth...
Embodiment 1
[0067] Add 10g of zinc powder and 60g of tetrahydrofuran into the reaction flask, add 19.3g of pentafluorobenzonitrile, add dropwise 3 drops of methanesulfonic acid, heat to reflux under nitrogen protection, slowly add 25g of ethyl bromoacetate dropwise, and reflux for 3 hours. Cool down to 20°C, add 80g of 20% hydrochloric acid dropwise, heat up to 70°C for hydrolysis and decarboxylation, and stir for 5 hours to complete the reaction. The temperature was raised for steam distillation, the fractions at 90-110°C were collected, and then the fractions were distilled at atmospheric pressure, and 15.6g of yellow liquid was collected at 130-135°C, which was pentafluoroacetophenone with a GC content of 99.5%.
[0068] In a water bath at room temperature, add 6 g of tap water to the reaction bottle, start stirring, slowly add 12 g of 98% concentrated sulfuric acid, slowly add 60 g of formic acid, the internal temperature is about 30 ° C, and add 15.6 g of raw material pentafluoroaceto...
Embodiment 2
[0072] Add 40g of zinc powder and 200g of tetrahydrofuran into the reaction flask, add 58g of pentafluorobenzonitrile, add dropwise 1ml of methanesulfonic acid, heat to reflux under nitrogen protection, slowly add dropwise 90g of methyl bromoacetate, and reflux for 3 hours. The temperature was lowered to 20° C., 100 g of 30% hydrochloric acid was added dropwise, and the organic layer was separated after reacting for 1 hour. 100 g of 30% hydrochloric acid was slowly added to the organic layer, and then the temperature was slowly raised to 70° C. for decarboxylation reaction, and the reaction was completed after stirring for 5 hours. The organic solvent was distilled off by raising the temperature, followed by steam distillation to obtain 51.3 g of a yellow liquid, namely pentafluoroacetophenone, with a GC content of >99%.
[0073] Put 50g of pentafluoroacetophenone and 250g of formic acid into the reaction kettle, turn on mechanical stirring and heat to 55-60°C, start to slowly...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com