Preparation method of acetic acid 2-hydroxyl-3-chloro-propyl ester compound
A technology of acetic acid and hydroxyl group, applied in the field of preparation of acetate compounds, can solve problems such as high production cost, equipment corrosion, environmental pollution of waste water, etc., and achieve the effects of easy product separation, low production cost and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] A kind of preparation method of acetic acid 2-hydroxyl-3-chloro-propyl ester, this method is raw material with acetic acid and epichlorohydrin, under the catalysis of phase transfer catalyst, reaction prepares acetic acid 2-hydroxyl-3-chloro-propyl ester, The molar ratio of acetic acid and epichlorohydrin is 1:1.02-1.05, and the catalyst is added. The reaction temperature is controlled at 105-110°C for 3 hours, and the temperature is controlled at 115-125°C for 3 hours. The acid value is less than 5mg / gKOH , and then evacuated at 100-105°C for 2 hours at a pressure of -0.04-0.06MPa to obtain high-purity 2-hydroxy-3-chloro-propyl acetate. Wherein said catalyzer is a phase transfer catalyst, preferably quaternary ammonium salt but not limited to: bisdecyldimethylammonium bromide, benzyltriethylammonium chloride in quaternary ammonium salt, consumption is 2-2 of the total amount of this step 3%.
[0025] The reaction temperature is divided into two temperature control pro...
Embodiment 1
[0033] Embodiment 1 Synthetic acetic acid 2-hydroxyl-3-chloro-propyl ester
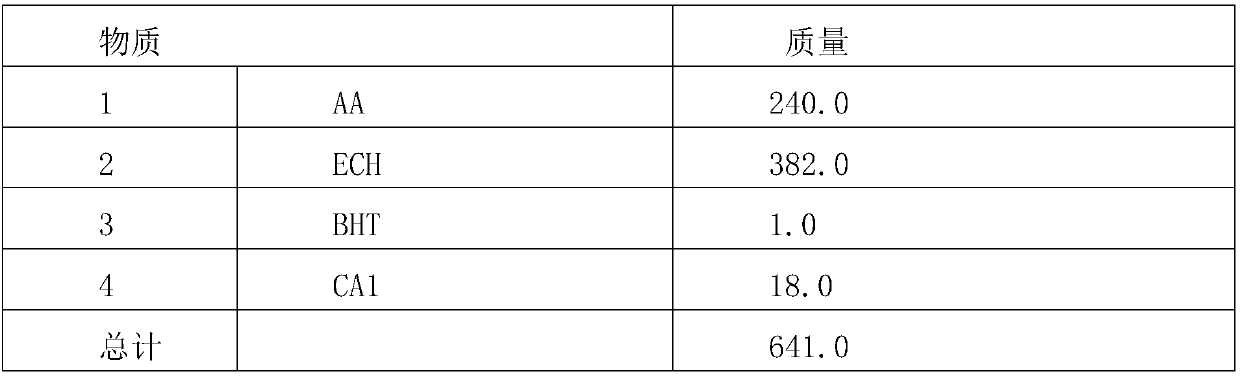
[0034] Table 1, synthetic formula table
[0035]
[0036] Add components 1, 2, 3, and 4 into the flask, heat and control the temperature at 105-110°C for 3 hours, then control the temperature at 115-125°C for 4 hours and then detect that the acid value is less than 5mg / gKOH , and then evacuated at 100-105°C for 2 hours at a pressure of -0.04-0.06MPa to obtain the target product 2-hydroxyl-3-chloro-propyl acetate.
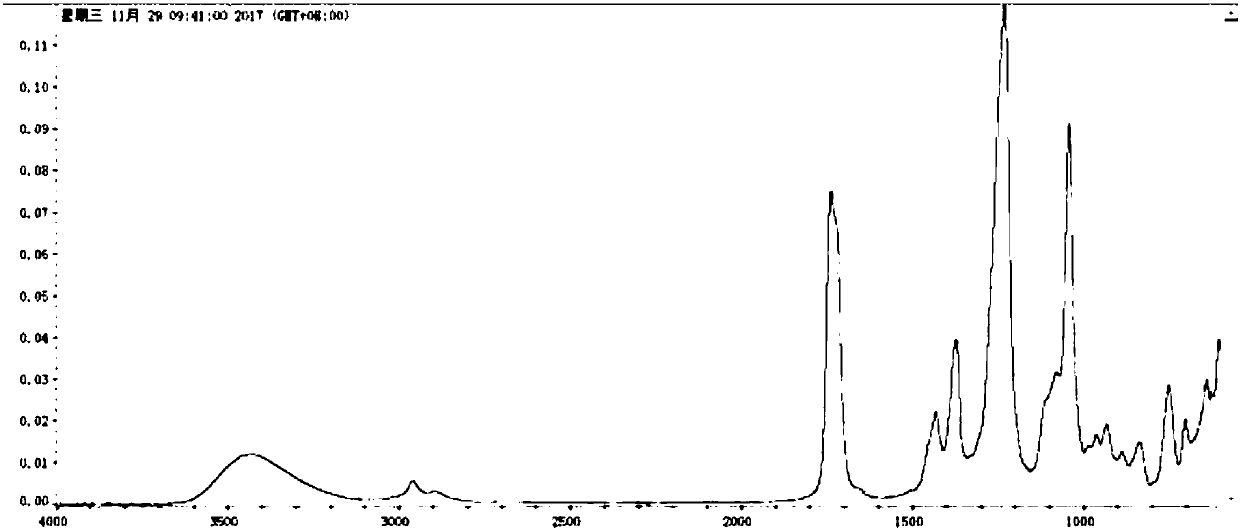
[0037] Product index: colorless liquid, viscosity---15cps / 25℃, refractive index---1.4582(23℃).
[0038] The infrared spectrum of the product is shown in figure 1 .
Embodiment 2
[0039] Embodiment 2 Synthetic acetic acid 2-hydroxyl-3-chloro-propyl ester
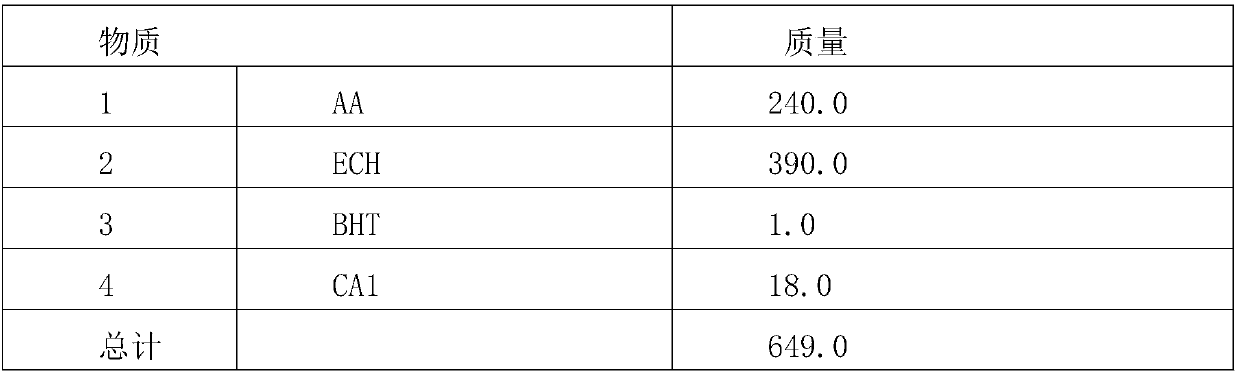
[0040] Table 2, synthetic formula table
[0041]
[0042] Add components 1, 2, 3, and 4 into the flask, heat and control the temperature at 105-110°C for 3 hours, then control the temperature at 115-125°C for 4 hours and then detect that the acid value is less than 5mg / gKOH , and then evacuated at 100-105°C for 2 hours at a pressure of -0.04-0.06MPa to obtain the target product 2-hydroxyl-3-chloro-propyl acetate.
[0043] Product index: colorless liquid, viscosity---15cps / 25℃, refractive index---1.4582(23℃).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com