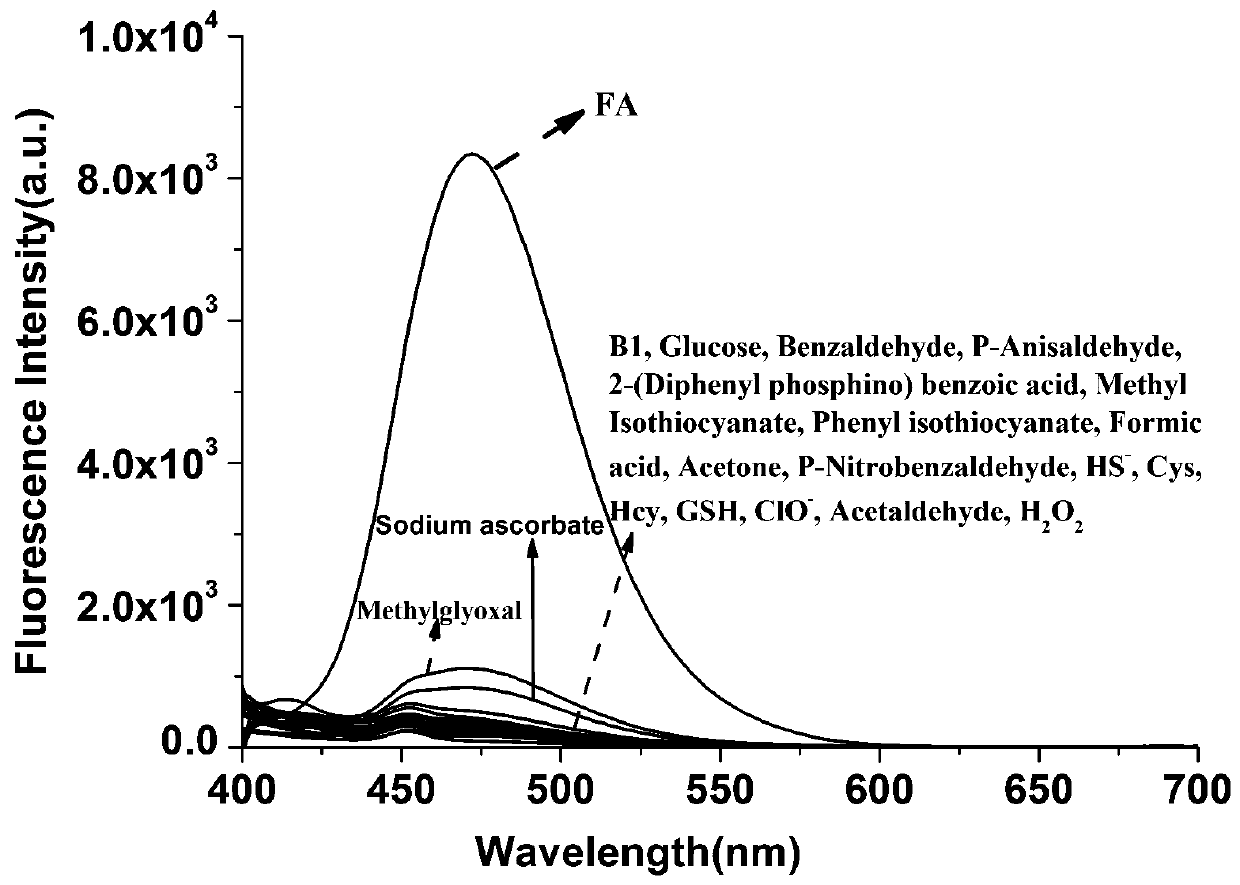
Pyrene derivative fluorescent probe molecule for recognizing and detecting formaldehyde as well as preparation method and application of fluorescent probe molecule
A technology of pyrene derivatives and fluorescent probes is applied in the field of identifying and preparing pyrene derivatives fluorescent probe molecules for detecting formaldehyde, which can solve the problems of high cytotoxicity, complicated preparation methods, inability to use formaldehyde detection agents, etc. The effect of low toxicity, simple and easy-to-obtain raw materials, and high fluorescence quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A preparation method for a pyrene derivative fluorescent probe molecule that recognizes and detects formaldehyde:
[0032] In a one-necked flask, potassium allyl trifluoroborate (148 mg, 1 mmol) was dissolved in 12.4 mL of ammonia-methanol solution (concentration: 7 mol / L), and stirred at room temperature for 15 min under nitrogen protection. Dissolve 1-pyrene formaldehyde (115 mg, 0.5 mmol) in 19.2 mL of ammonia-methanol solution (concentration: 7 mol / L), add 115 μL of water, and inject the reaction solution into the allyl potassium trifluoroborate Ammonia-methanol solution was reacted at room temperature for 10 h. After the reaction was completed, the solvent was removed under reduced pressure by a rotary evaporator to obtain a crude product. After separation by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:3, volume ratio), 88 mg of yellow solid was obtained as product B1, and the yield was 65%.
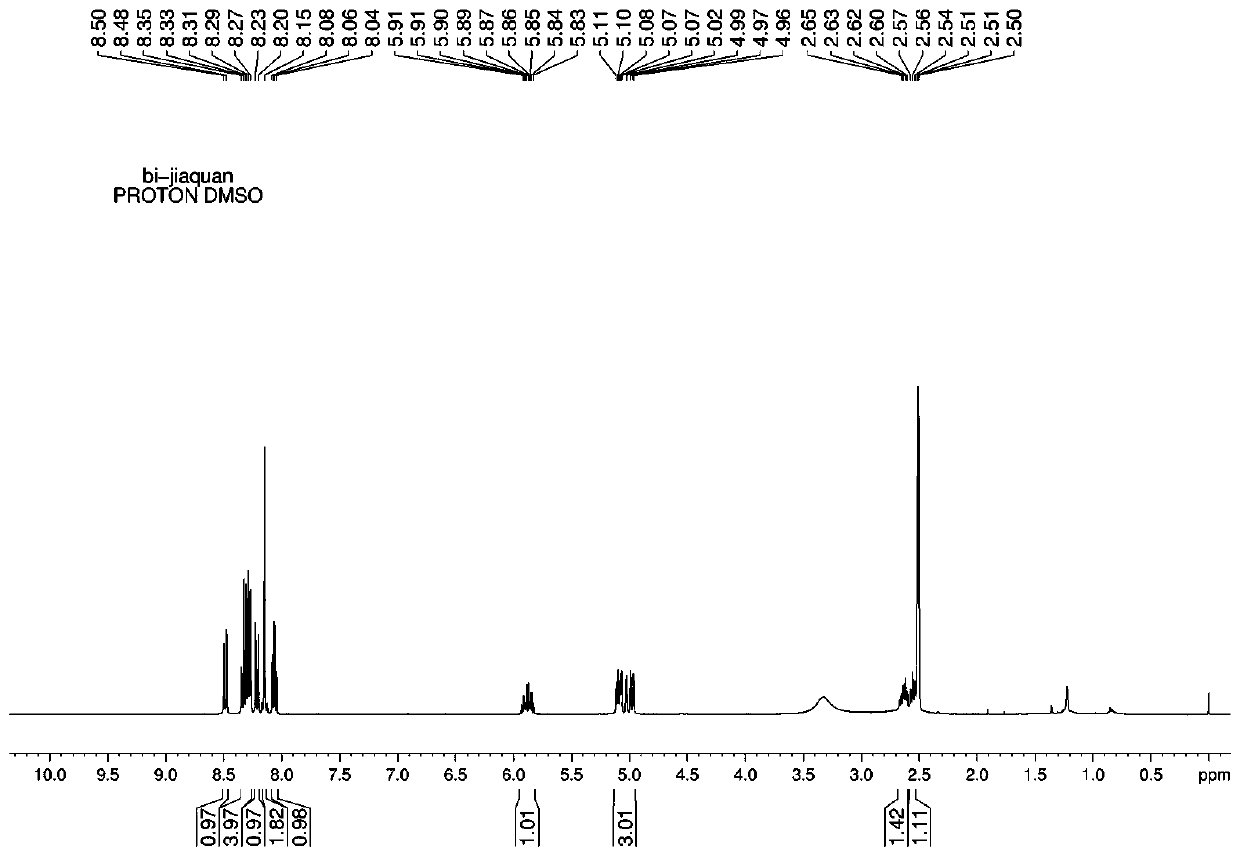
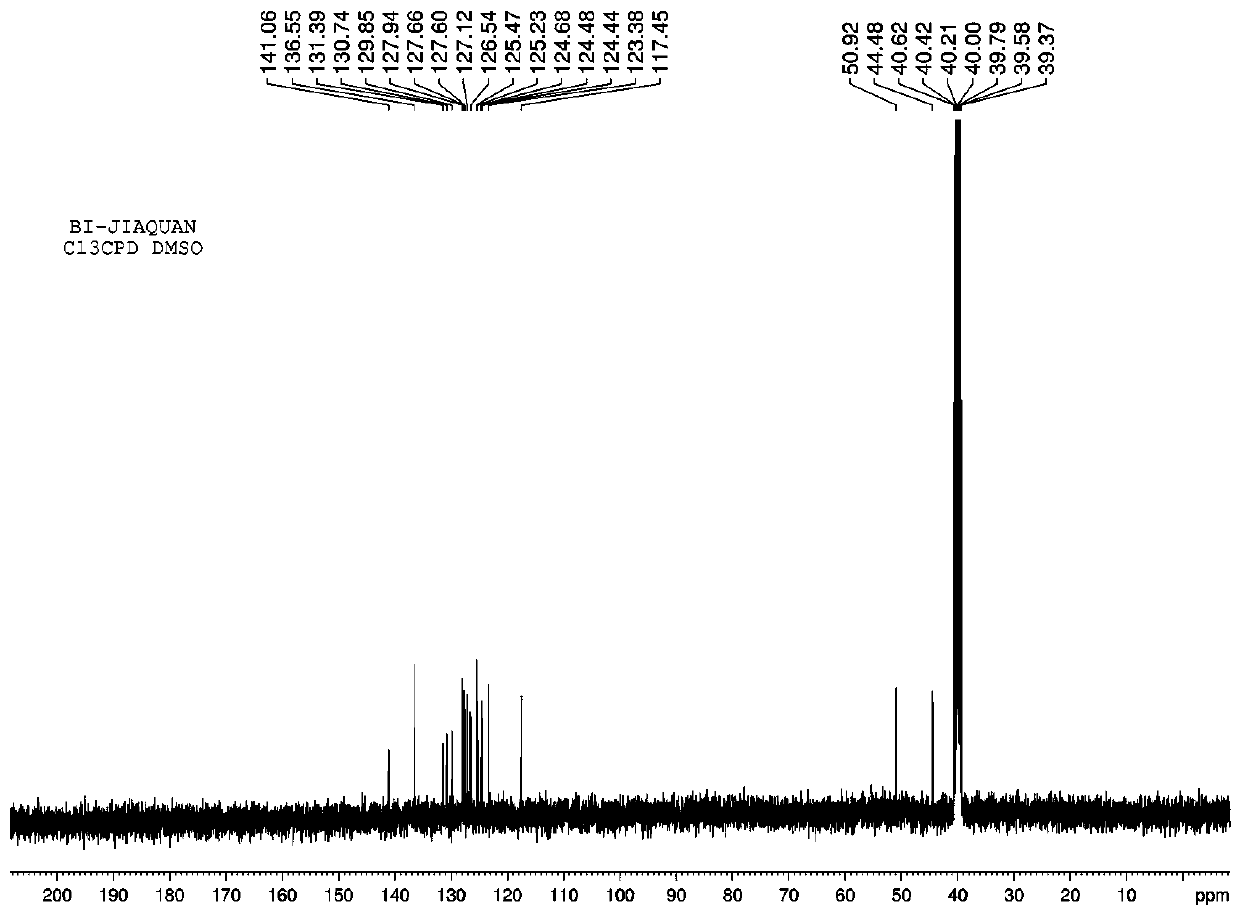
[0033] NMR measurement: 1H NMR (CDCl3, 4...
Embodiment 2
[0035] A preparation method for a pyrene derivative fluorescent probe molecule that recognizes and detects formaldehyde:
[0036] In a one-necked flask, potassium allyl trifluoroborate (148 mg, 1 mmol) was dissolved in 43.4 mL of ammonia-methanol solution (concentration: 7 mol / L), and stirred at room temperature for 15 min under nitrogen protection. Dissolve 1-pyrenecarbaldehyde (57.5 mg, 0.25 mmol) in 19.2 mL of ammonia-methanol solution (concentration: 7 mol / L), add 12 μL of water, and inject the reaction solution into the allyl potassium trifluoroborate Ammonia-methanol solution was reacted at room temperature for 15 h. After the reaction was completed, the solvent was removed under reduced pressure by a rotary evaporator to obtain a crude product. After separation by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:6, volume ratio), 50.8 mg of yellow solid was obtained as product B1, and the yield was 75%.
[0037]NMR measurement: 1H NMR (CDCl3, ...
Embodiment 3
[0039] A preparation method for a pyrene derivative fluorescent probe molecule that recognizes and detects formaldehyde:
[0040] In a one-necked flask, potassium allyl trifluoroborate (148 mg, 1 mmol) was dissolved in 74.4 mL of ammonia-methanol solution (concentration: 7 mol / L), and stirred at room temperature for 15 min under nitrogen protection. Dissolve 1-pyrene formaldehyde (28.8 mg, 0.13 mmol) in 19.2 mL of ammonia-methanol solution (concentration: 7 mol / L), add 12 μL of water, and inject the reaction solution into the allyl potassium trifluoroborate Ammonia-methanol solution was reacted at room temperature for 20 h. After the reaction was completed, the solvent was removed under reduced pressure by a rotary evaporator to obtain a crude product. After separation by silica gel column chromatography (eluent: ethyl acetate:petroleum ether=1:6, volume ratio), 30.5 mg of yellow solid was obtained as product B1, and the yield was 90%.
[0041] NMR measurement: 1H NMR (CDCl3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com