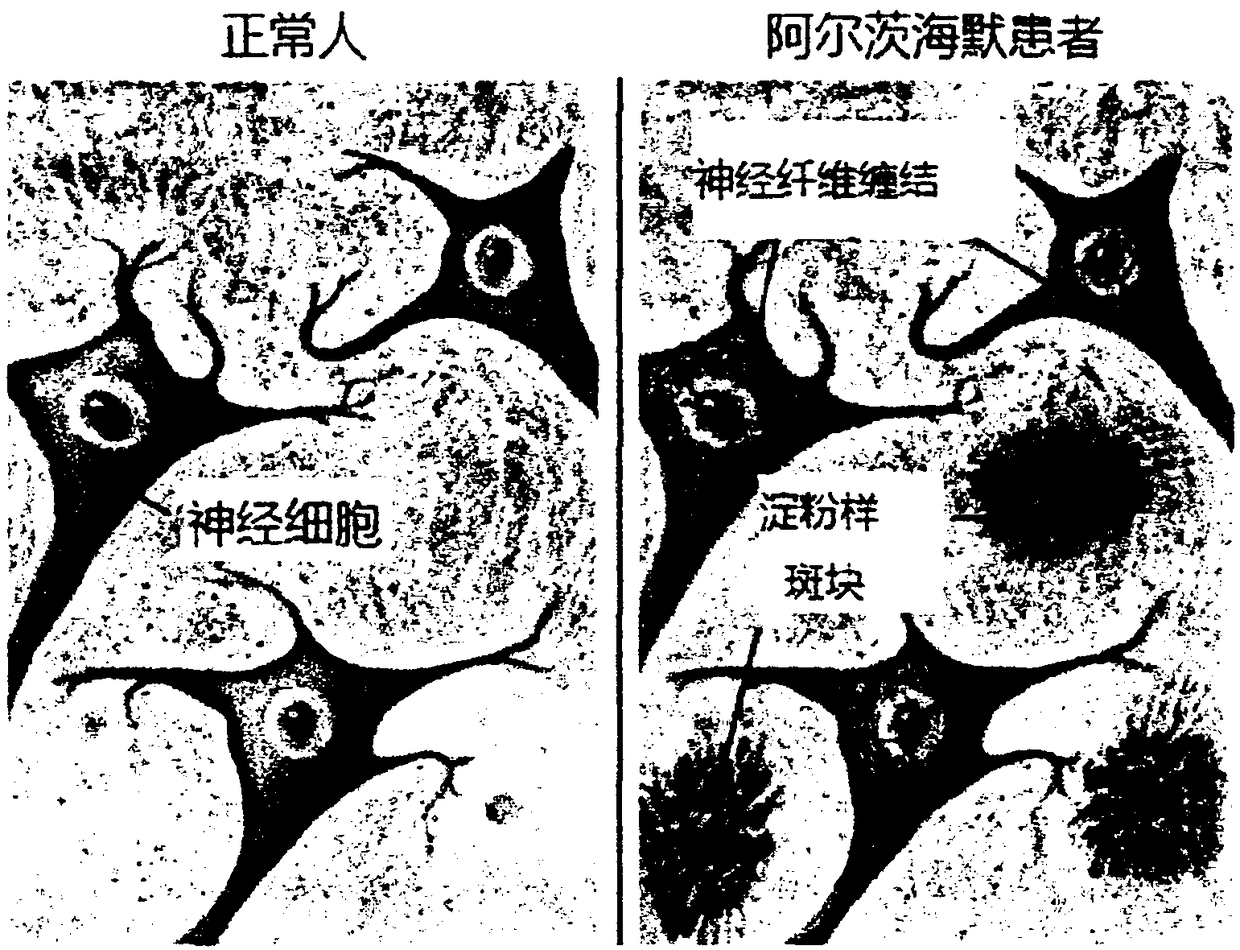
Amyloid protein-labeled fluorescence probes, and making method and application thereof
A fluorescent probe and reaction technology, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., to achieve the effect of biological activity advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1C3
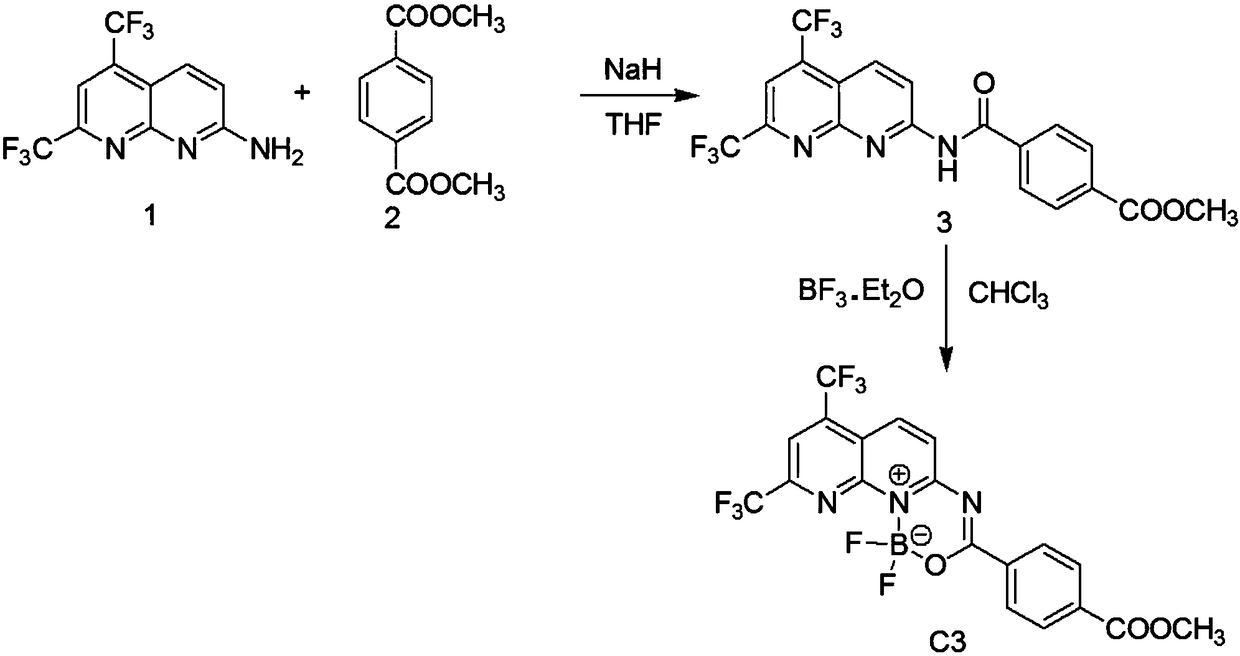
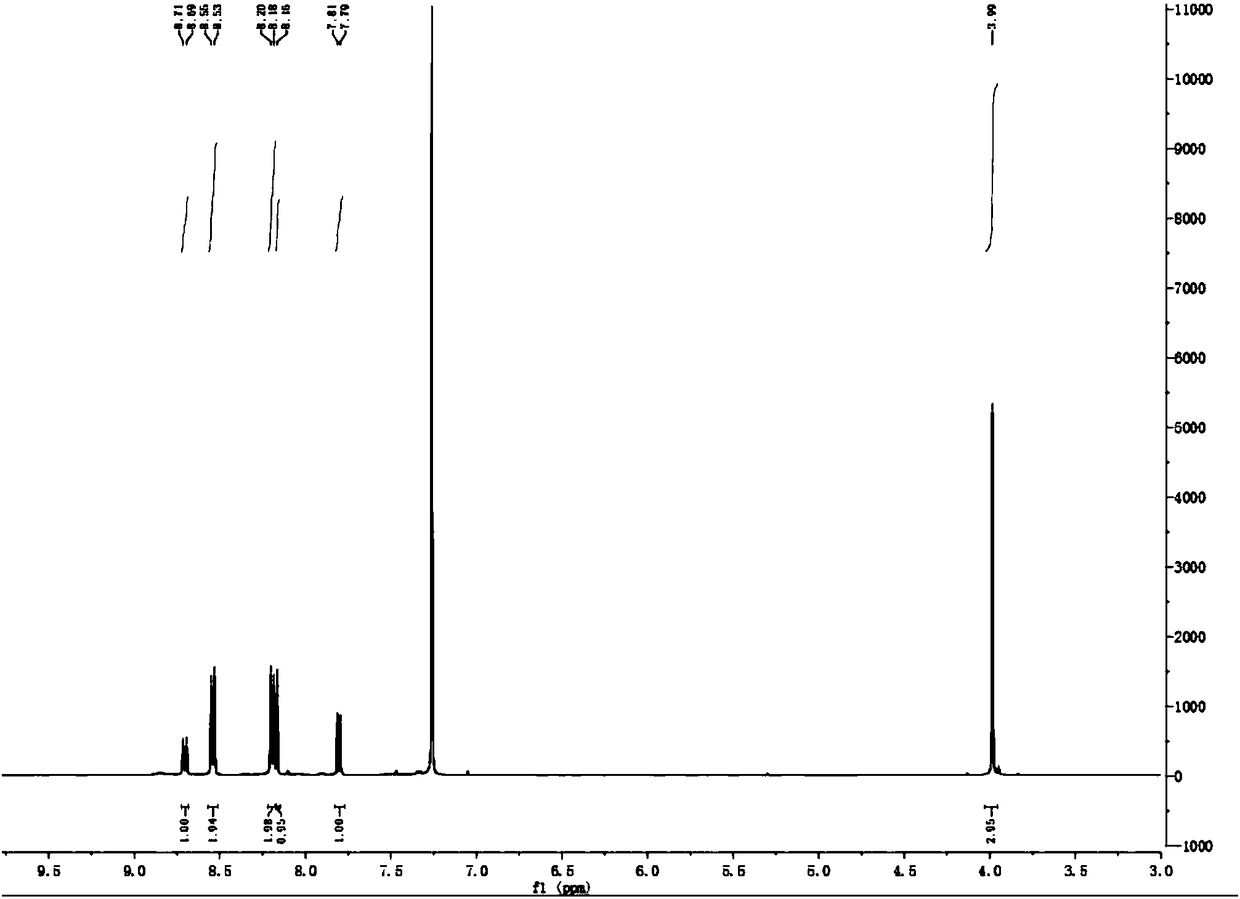
[0049] The preparation of embodiment 1C3 compound
[0050] according to figure 2 The synthetic scheme shown prepares the C3 compound.
[0051] step 1:
[0052] Synthesis of 2-amino-5,7-trifluoromethyl-1,8-naphthyridine (compound 1): 2,6-diaminopyridine (commercially available, analytically pure, 10.9g, 0.1mol) and hexafluoroacetyl Acetone (commercially available, analytically pure, 10.0 g, 0.1 mol), 100 mL of phosphoric acid, under nitrogen protection, stirred and refluxed for 24 hours. The reaction solution was cooled, and adjusted to neutrality with ammonia water under ice bath conditions. Refrigerate overnight at 5°C, then filter with suction, wash the filter cake with cold water, and dry to obtain the crude product. The crude product was recrystallized from absolute ethanol to obtain light yellow solid powder. Yield: 17.3g (61.3%).
[0053] Step 2:
[0054] Synthesis of Compound 3: Compound 1 (2.81 g, 0.01 mol) and Compound 2 (0.01 mol) were dissolved in 100 mL of an...
Embodiment 2
[0061] Example 2 Compound C3 is used for amyloid Aβ 42 , Aβ 40 fluorescent label
[0062] 1. Amyloid Aβ 42 , Aβ 40 Preparation of Polymers: Human Amyloid Amyloid Aβ 42 (AS-20276, purified by HPLC, AnaSpec Co.), Aβ 40 (AS-24236, purified by HPLC, AnaSpec Co.) 1mg was dissolved in 4mL PBS buffer solution (pH=7.4), the protein concentration was 0.25mg / mL, continued to shake gently, incubated at 37°C for 42h, added PBS buffer solution to dilute 0.2 mg / mL;
[0063] 2. Compound C3 ethanol solution (1 μmol, spectrum pure) 300 μL, add Aβ 42 polymer or Aβ 40 Polymer or BSA (bovine serum albumin, 0.2mg / mL, PBS buffer solution) 300μL, then add PBS buffer solution 2400μL, the total solution volume is 3000μL, the total protein is 60μg, the preparation ratio is compound C3: protein 1:1, The mixture was incubated at room temperature for 30 min;
[0064] 3. Fluorescence detection after incubation, fluorescence instrument F-4600FL Spectrophotometer, EX WL: 350nm.
[0065] Figure 5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com