Biomarkers for the diagnosis of endometriosis
A purpose and specific technology, applied in the field of medical diagnosis, to facilitate staining, avoid contamination, and improve sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Collection of endometrial cell samples
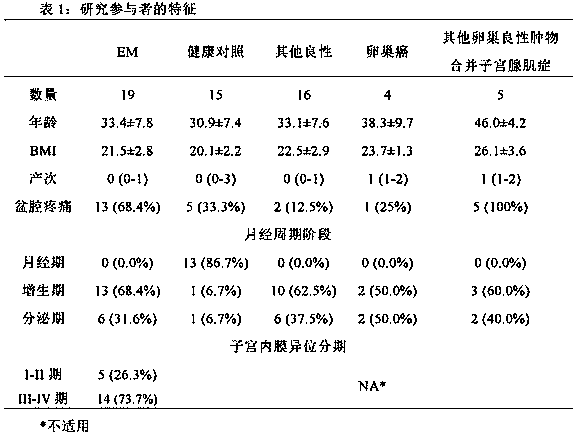
[0060] A total of 59 patients were recruited. Forty-four patients underwent surgical treatment at Peking University People's Hospital from October 2015 to July 2016 due to ovarian tumors detected by ultrasound. According to laparoscopic and pathological diagnosis, 19 cases were EM, 25 cases were non-EM (including 16 cases of other benign ovarian tumors, 4 cases of ovarian cancer, and 5 cases of other benign ovarian tumors complicated with adenomyosis (Table 1). Among them, the age of EM group was 33.4±7.8 years (22-45 years old), BMI was 21.5±2.8 (17.4-26.7), 13 cases (68.4%) had dysmenorrhea, 13 cases (68.4%) were in proliferative stage, 6 cases (31.6% %) was in the secretory phase, and the EM stage was judged according to rAFS (revised Classification of the American Society of Reproductive Medicine). Among them, 5 cases (26.3%) were in stage I-II, and 14 cases (73.7%) were in stage III-IV. In addition, 15 cases were...
Embodiment 2
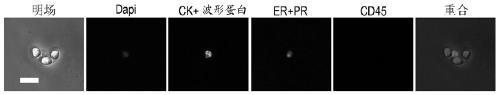
[0064] Example 2: Detection of endometrial cells
[0065] The cell suspension collected in Example 1 was injected into the microfluidic chip (Lv P et al. , 2013), endometrial cells were captured by micropillars arranged in a spatial gradient. The endometrial cells were identified in the chip by immunofluorescent staining. Inject 4% paraformaldehyde into the chip to fix the cells for 20 minutes. After washing with PBS, pass through 0.1% Triton X-100 (Sigma) for 10 minutes to rupture the membrane. After washing with PBS, block with 10% bovine serum (Wisent) for 20 minutes, and then inject Primary antibody and DAPI (Life Technologies), incubated overnight at 4°C, washed with PBS, injected with secondary antibody and incubated at room temperature. Images were observed and collected using a fluorescent inverted microscope (DM IL LED, Leica Microsystems). The primary antibodies were used as follows: pre-stained PE anti-Vimentin mouse monoclonal antibody (1:100, Abcam), pre-stain...
Embodiment 3
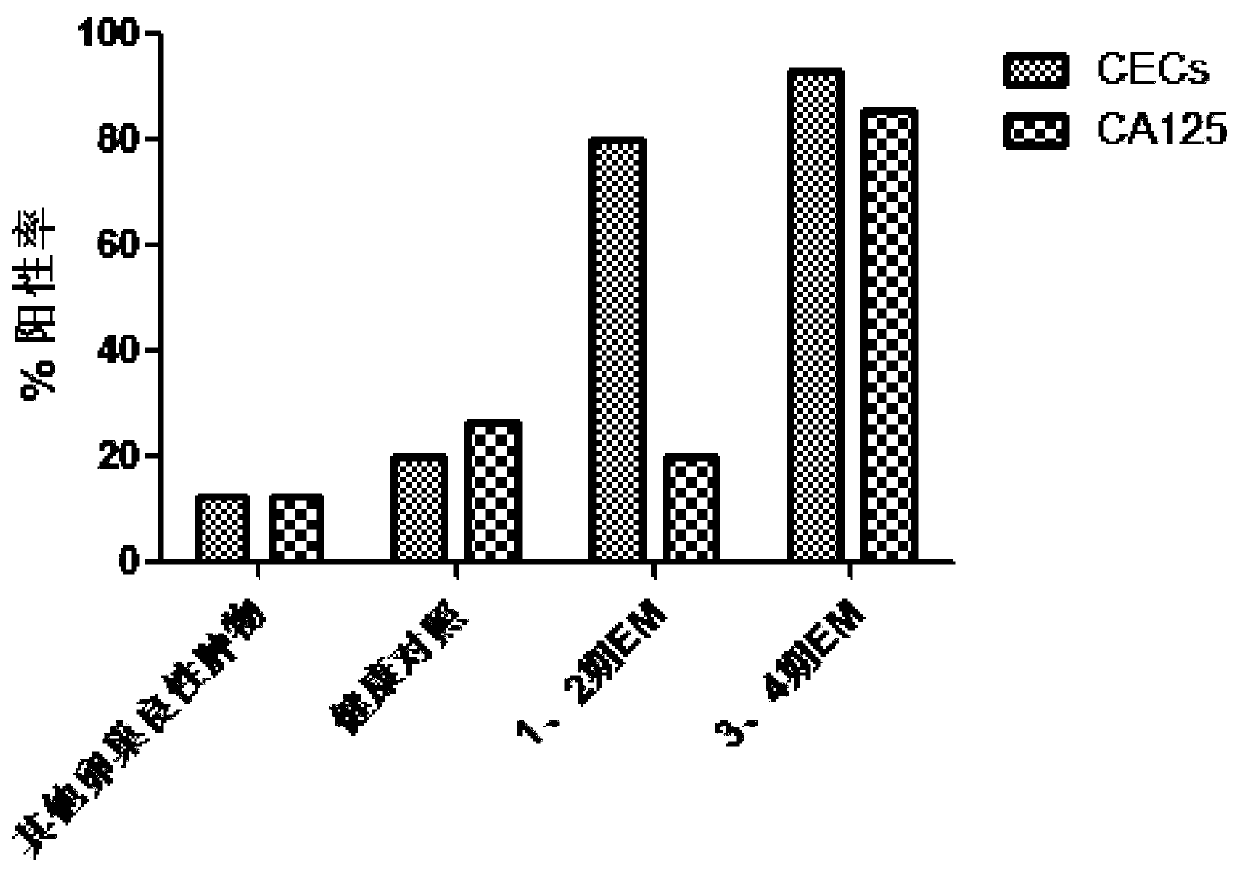
[0073] Example 3: The value of CECs in auxiliary diagnosis of EM
[0074] In order to evaluate whether CECs can be used as EM biomarkers for auxiliary diagnosis, we calculated the sensitivity and specificity of CECs when compared with other benign tumor groups and healthy controls, and compared with serum CA125 (>35U / mL defined as test positive) were compared ( figure 2 ).
[0075] Serum CA125 assays from the same subjects in Example 1 were used as controls. Serum CA125 was quantitatively detected by the Laboratory Department of Peking University People's Hospital using the electrochemiluminescence method, and using the Carbohydrate Antigen 125 Detection Kit (Roche Diagnostics) in accordance with the operating procedures given by the manufacturer. The detection system is Cobase 411x electrochemiluminescence automatic immunoassay system (Roche Diagnostics)
[0076] Statistical analysis was performed with SPSS22 software. Normality testing of the data was performed by the K...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com