Catalyst with shell-core structure as well as preparation method and application of catalyst
A core-shell structure and catalyst technology, applied in the field of catalysts and their preparation, can solve the problems of reducing the selectivity of methyl halides and low yields of methyl halides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
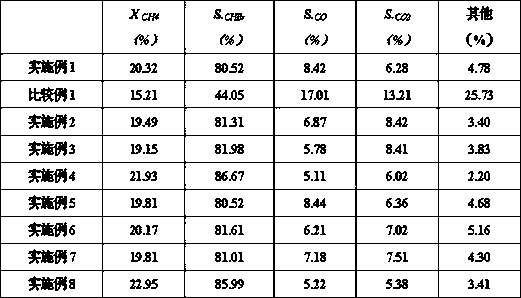
Examples
Embodiment 1
[0024] Prepare aluminum hydroxide slurry by hydrolysis of aluminum isopropoxide: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80°C-85°C, hydrolyze aluminum isopropoxide for 1.5h, and then carry out aging , the aging temperature was controlled at 90°C-95°C, the aging time was 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% was obtained.
[0025] Preparation of zinc-loaded alumina: use equal volume impregnation method on alumina (commercially available, the properties are as follows: L acid content is 0.2mmol g -1 ; BET specific surface is 200; pore volume is 0.3ml / g) impregnated with zinc nitrate solution, dried and roasted after impregnation, the drying time is 2h, and the drying temperature is 130°C; the roasting time is 4h, and the temperature is 400°C , the zinc-loaded alumina is spherical, and the equivalent diameter of the zinc-loaded alumina is 2 mm;
[0026] Spray immersion process: mix an ap...
Embodiment 2
[0030] Prepare aluminum hydroxide slurry by hydrolysis of aluminum isopropoxide: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80°C-85°C, hydrolyze aluminum isopropoxide for 1.5h, and then carry out aging , the aging temperature was controlled at 90°C-95°C, the aging time was 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% was obtained.
[0031] Preparation of zinc-loaded alumina: use equal volume impregnation method on alumina (commercially available, L acid content is 0.1mmol g -1 ;BET specific surface is 300m 2 / g; the pore volume is 0.5ml / g) impregnated with zinc sulfate solution, dried and roasted after immersion, the drying time is 3h, and the drying temperature is 120°C; the roasting time is 5h, the temperature is 450°C, the load Zinc alumina is spherical, and the equivalent diameter of zinc-loaded alumina is 2mm;
[0032] Spray immersion process: mix an appropriate amount of zirconium sulfat...
Embodiment 3
[0036] Prepare aluminum hydroxide slurry by hydrolysis of aluminum isopropoxide: mix water and aluminum isopropoxide at a molar ratio of 120:1, control the hydrolysis temperature at 80°C-85°C, hydrolyze aluminum isopropoxide for 1.5h, and then carry out aging , the aging temperature was controlled at 90°C-95°C, the aging time was 18h, and an aluminum hydroxide slurry with a solid content of 21.3wt% was obtained.
[0037] Preparation of zinc-loaded alumina: using an equal volume impregnation method on alumina (commercially available, the properties are as follows: the content of L acid is 0.05 mmol g -1 ; BET specific surface is 250m 2 / g; the pore volume is 0.3-0.6ml / g, preferably 0.4ml / g,) impregnated with zinc bromide solution, dried and roasted after impregnated, the drying time is 4h, and the drying temperature is 100°C; The calcination time is 4 hours, the temperature is 500°C, the zinc-loaded alumina is spherical, and the equivalent diameter of the zinc-loaded alumina i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com