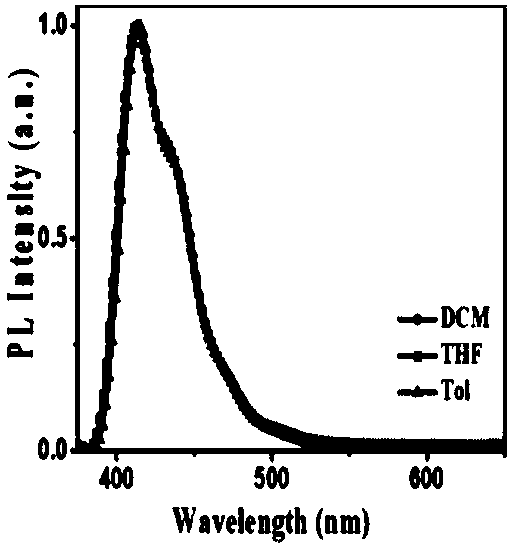
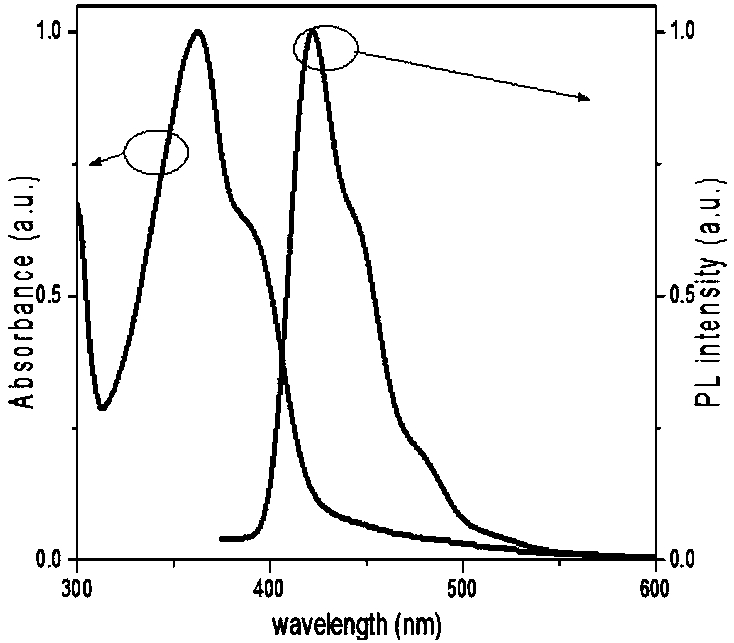
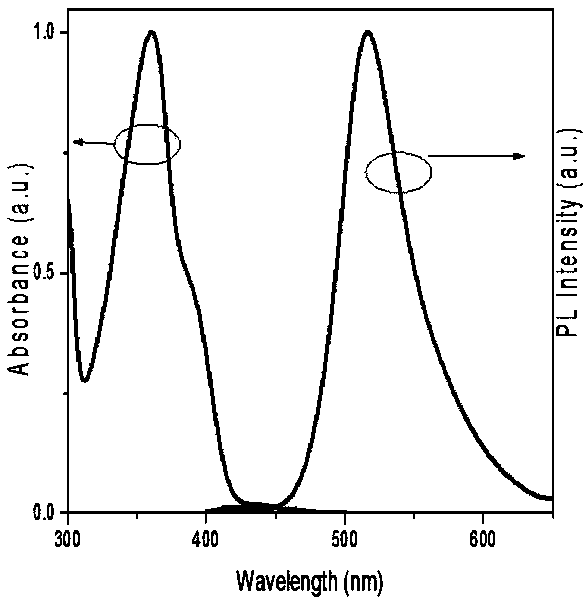
Poly(spirobifluorene) and organic light-emitting device
A polyspirofluorene and selected technology, which is applied in the field of polyspirofluorene and organic electroluminescent devices, can solve the problems of polyspirofluorene without charge transfer, decrease in fluorescence quantum efficiency, prolong fluorescent life and the like, and achieve good hole efficiency. Transmission capability, effect of good device efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0119] The invention provides a preparation method of polyspirofluorene, comprising:
[0120] The dihalogen monomer with the structure of formula (II) and the diboron derivative monomer with the structure of formula (III) are polymerized in the presence of a palladium compound, a basic compound, an organic phosphine compound, a solvent, and a catalyst to obtain polyspirofluorene;
[0121] Or the dihalogen monomer of the formula (II) structure, the diboron derivative monomer of the formula (III) structure and the aromatic compound are polymerized in the presence of a palladium compound, a basic compound, an organic phosphine compound, a solvent, and a catalyst to obtain a polymer Spirofluorene;
[0122]
[0123] Among them, R 1 , R 2 , R 3 , R 4 Independently selected from C1-C22 straight-chain alkyl, C1-C22 branched-chain alkyl, C3-C22 cycloalkyl, C1-C22 alkoxy or C1-C22 heteroalkyl;
[0124] M is selected from one of chlorine trifluoromethanesulfonate and halogen; B i...
Embodiment 1
[0169] Carbazole (50g, 0.3mol), AlCl 3 (88.0g, 0.66mol) and dry dichloromethane (300mL) were added to a 1L three-necked flask, and mechanical stirring was turned on to dissolve; Pass into the sodium hydroxide solution; after the dropwise addition, the temperature is raised to reflux at 45°C, and the reaction is carried out for 12 hours. 3M HCl (300mL) solution was added dropwise in the system, and the solvent dichloromethane was distilled off to obtain a brown solid washed with a large amount of distilled water to obtain 82.8g of 3,6-bis(1-octanoyl)carbazole powder. 65.8%. The purity is 99.0%. The product obtained is carried out nuclear magnetic analysis, and the result is: 1 HNMR (400MHz, CDCl 3 ,δ):8.79(d,J=1.1Hz,2H),8.61(s,1H),8.15(dd,J=8.5,1.5Hz,2H),7.50(d,J=8.6Hz,2H),3.11 (t, J = 7.5Hz, 4H), 1.88–1.75 (m, 4H), 1.49–1.25 (m, J = 47.7, 8.0Hz, 16H), 0.90 (t, J = 6.9Hz, 6H).
Embodiment 2
[0171] The 3,6-di(1-octanoyl)carbazole powder (57.0g, 136mmol) obtained in Example 1, hydrazine hydrate (132mL, 2.17mol), sodium hydroxide (54.4g, 1.36mol) and a Diethylene glycol (500mL) was added to a 1L three-necked flask, and the three necks of the flask were respectively equipped with a mechanical stirring device, a built-in thermometer and a water separator; the stirring was turned on, the temperature was raised to 110°C for 4 hours, the temperature was raised to 150°C for 12 hours, and the temperature was raised to React at 190°C for 6h, release the generated gas and moisture, then raise the temperature to 210°C for 6h. After the reaction was completed, the system was cooled to room temperature, poured into a large amount of water, filtered, and the filter cake was drained. Using 200-300 mesh silica gel as the stationary phase and dichloromethane as the eluent, 43 g of light yellow solid 3,6-dioctylcarbazole was obtained by column separation, with a yield of 65%. The p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Maximum luminescence wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com