Method for preparing N1-long chain alkane substituted-4,5-dimethylimidazole type basic anion exchange membrane
A dimethyl imidazole, basic anion technology, applied in climate sustainability, final product manufacturing, sustainable manufacturing/processing, etc., can solve the decline of electrical conductivity and mechanical strength, quaternary ammonium group detachment from the framework, and ionic conductivity. and other problems, to achieve the effect of being environmentally friendly, overcoming low conductivity, and resisting attacks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
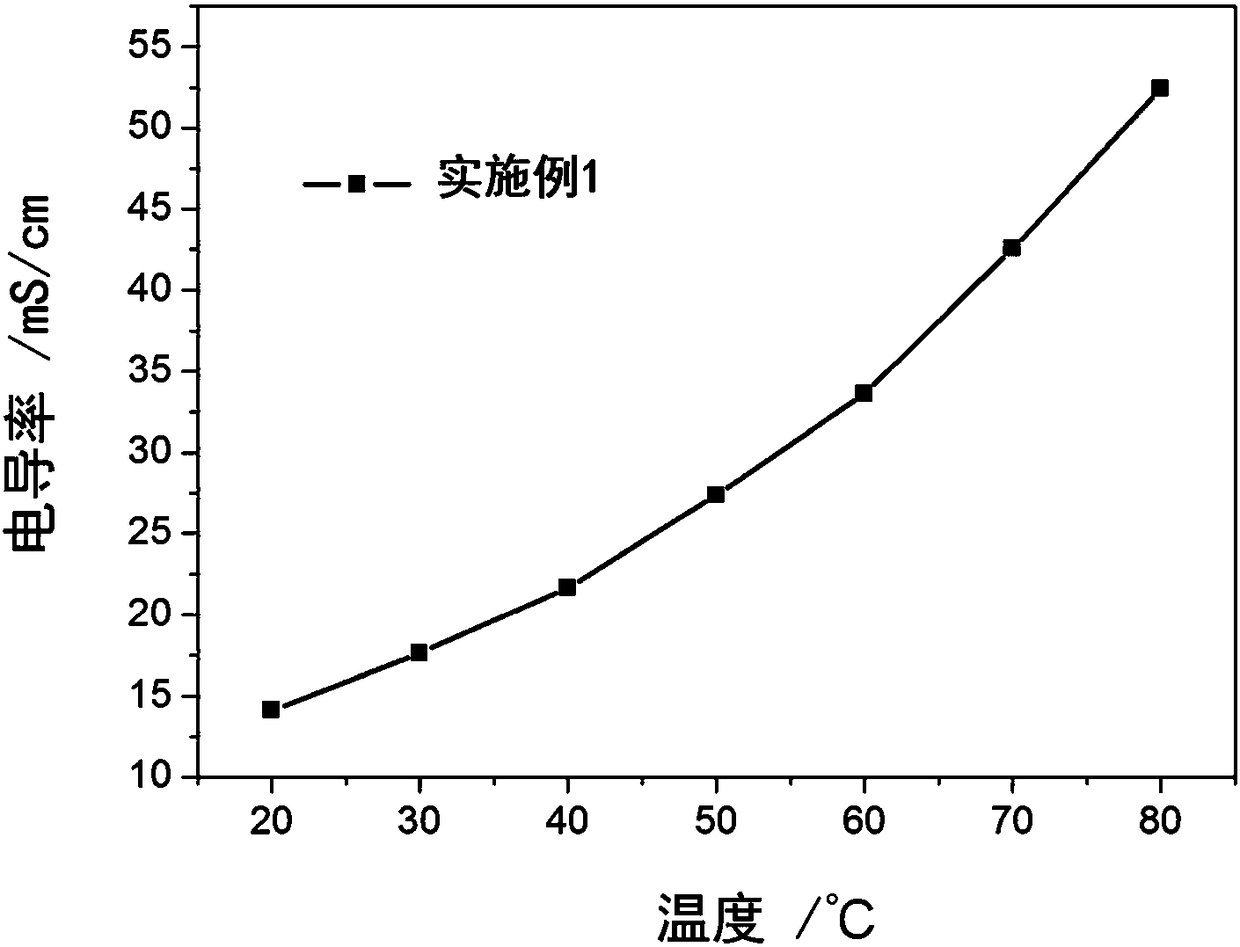
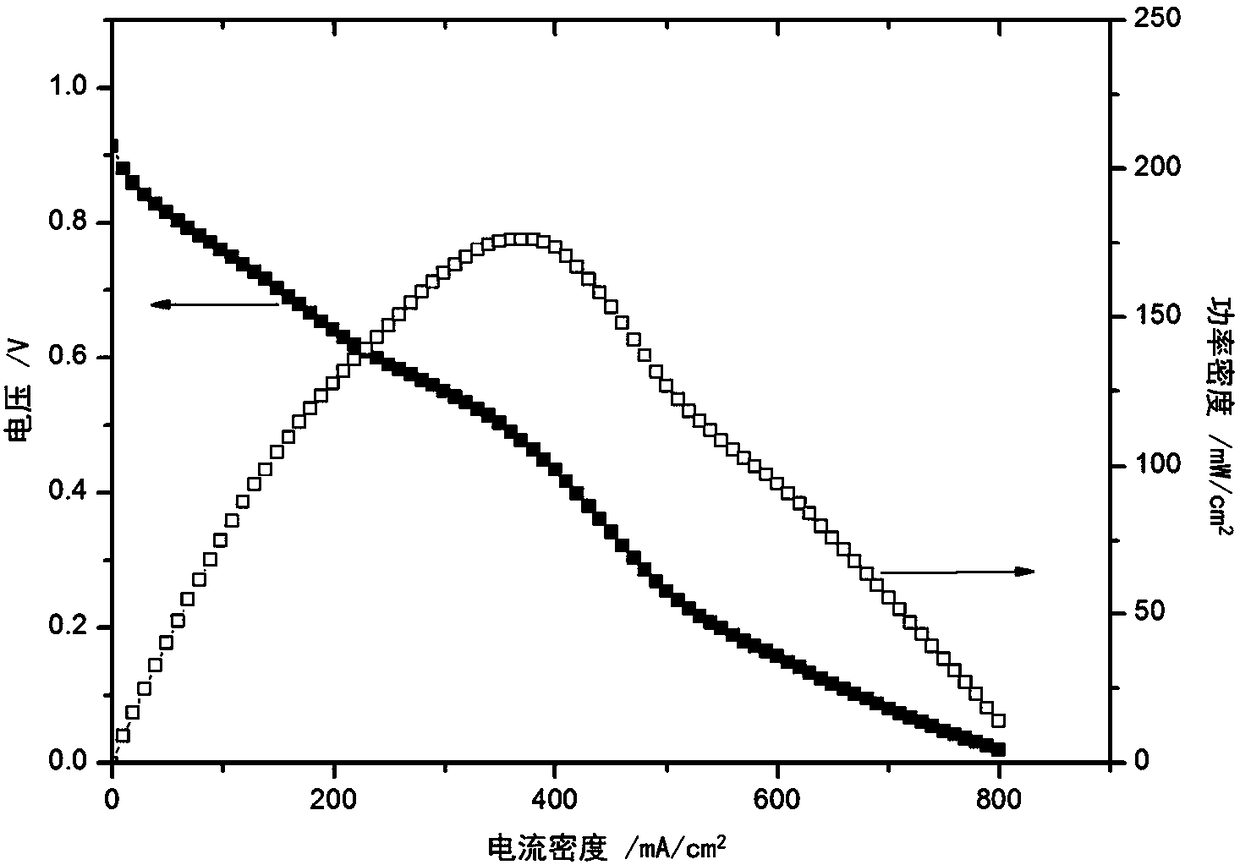
Embodiment 1
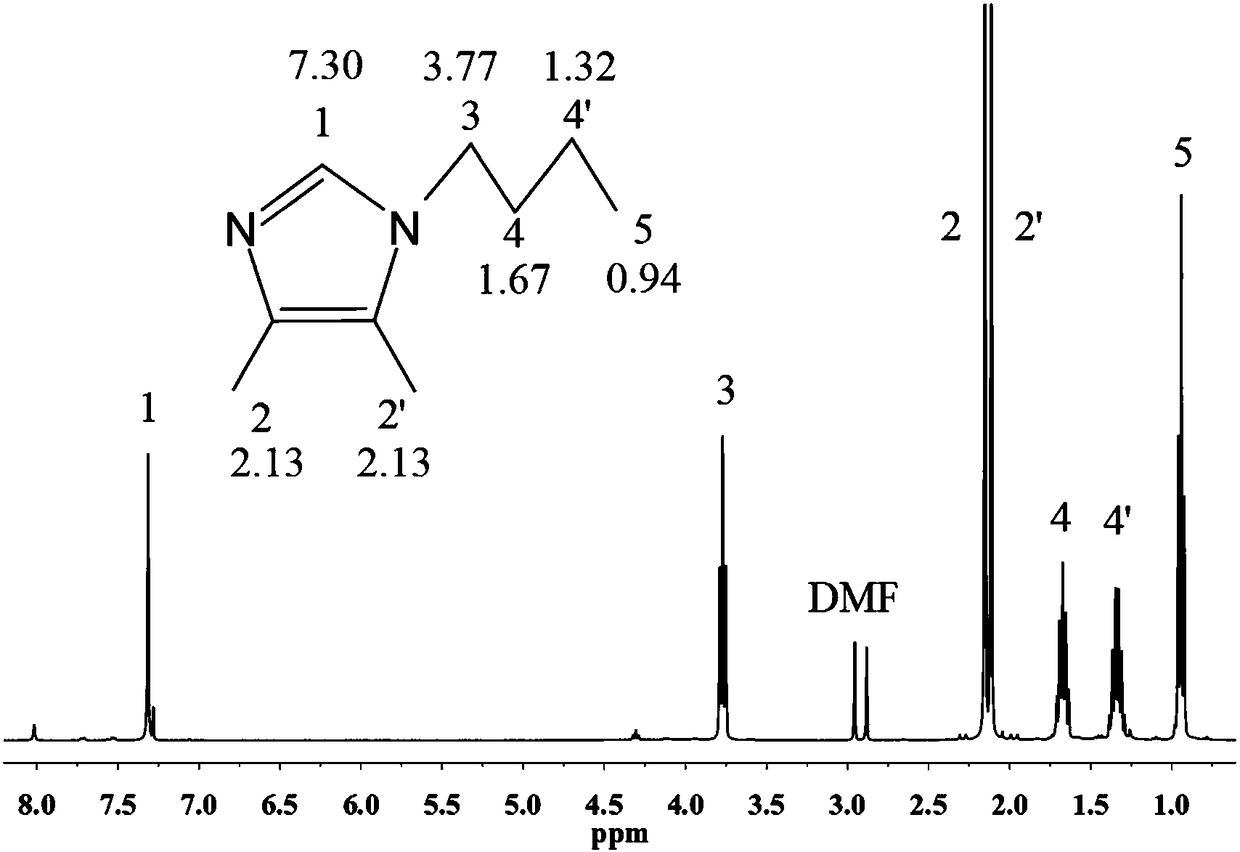
[0037] The synthesis process of 1-butyl-4,5-dimethylimidazolium salt is as follows,
[0038] 1) Add 2gNaH to 20mL NMP solvent, stir in an ice-water bath for 30 minutes, slowly add 2.54,5-dimethylimidazolium salt while stirring, after stirring for 1h, the solution becomes a dark brown turbid liquid;
[0039] 2) Slowly add brominated n-butane alkane dropwise to the solution, the amount of which is 1.5 times the molar mass of 4,5-dimethylimidazolium salt, and stir and react at 30°C for 12 hours to obtain a dark yellow opaque liquid;
[0040] 3) Pour the reaction product into deionized water, and extract with ethyl acetate (ethyl acetate: water volume ratio is 1:1), the extract is a clear light yellow liquid, wash the extract with water several times, and distill under reduced pressure remove ethyl acetate;
[0041] 4) The crude product is separated and purified by chromatography, and the obtained product is 1-butyl-4,5-dimethylimidazole.
[0042] The preparation method of 1-but...
Embodiment 2
[0054] The synthesis process of 1-hexyl-4,5-dimethylimidazolium salt is as follows,
[0055] 1) Add 1gNaH to 20mL of DMAc solvent, stir in an ice-water bath for 30 minutes, slowly add 1.8g of 4,5-dimethylimidazolium salt while stirring, and after stirring for 1h, the solution becomes a dark brown turbid liquid;
[0056] 2) Slowly add n-bromohexane dropwise to the solution, the amount of which is 1.5 times the molar mass of 4,5-dimethylimidazolium salt, and stir and react at 50°C for 124 hours to obtain a dark yellow opaque liquid;
[0057] 3) Pour the reaction product into deionized water, and extract with ethyl acetate (ethyl acetate: water volume ratio is 1:1), the extract is a clear light yellow liquid, wash the extract with water several times, and distill under reduced pressure remove ethyl acetate;
[0058] 4) The crude product is separated and purified by chromatography, and the obtained product is 1-hexyl-4,5-dimethylimidazole.
[0059] The preparation method of 1-he...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Power density | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com