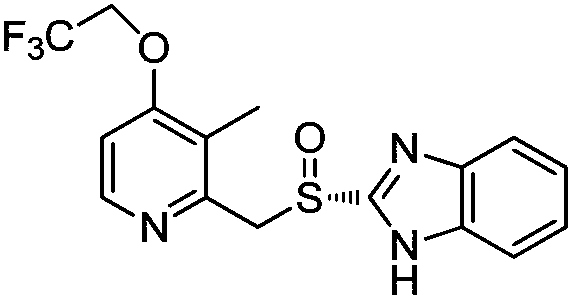
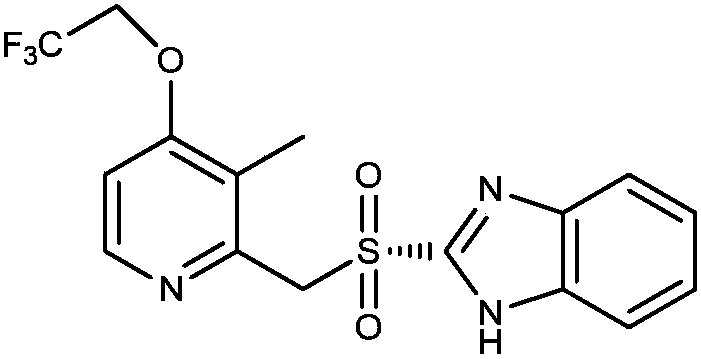
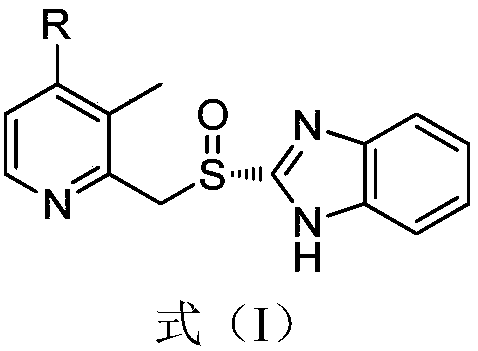
Preparation method of dexlansoprazole
A technology of dexlansoprazole and trifluoroethanol, applied in the field of pharmaceutical synthesis, can solve the problems of large sulfone-type impurities, high cost and low purity of the product, and achieve the effects of shortening the process cycle, improving the yield and simplifying the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040]Add dimethyl sulfoxide (DMSO)) 4L, trifluoroethanol 2.6kg, sodium trifluoroethoxide 2.5kg, R-2-(((4-chloro-3-methyl-2-pyridyl) Methyl)sulfinyl)benzimidazole 800g, heated to 90-100°C, reacted for 3-4 hours, HPLC detection formula (I) compound content was 0.04%, sulfone type impurity content 0.23%; the reaction solution was cooled to At room temperature, add 24L of 5% sodium chloride aqueous solution dropwise, lower to 0-10°C and stir for 0.5-1 hour, filter, dissolve the obtained solid in 24L of ammonia water, wash with dichloromethane (8L×3), stir for 15-20 Minutes, separate the liquid, adjust the pH of the ammonia water layer to 8-9 with glacial acetic acid (temperature control 0-10°C during the period), stir for 0.5-1 hour, filter, dissolve the obtained solid in 8L of acetonitrile, add 32L of water dropwise, and stir for 2-3 hours , filtered, the obtained solid was dissolved in 8L of acetonitrile again, 32L of water was added dropwise, stirred for 2-3 hours,...
Embodiment 2
[0043]
[0044] Add 1 L of N-methylpyrrolidone (NMP), 650 g of trifluoroethanol, 625 g of sodium trifluoroethoxide, R-2-(((4-chloro-3-methyl-2-pyridyl) methyl ) sulfinyl)benzimidazole 200g, heated to 90-100°C, reacted for 3-4 hours, HPLC detected that the compound content of formula (I) was 0.05%, and the content of sulfone-type impurities was 0.3%; the reaction solution was cooled to room temperature, Add 6L of 5% aqueous sodium chloride solution dropwise, drop to 0-10°C and stir for 0.5-1 hour, filter, dissolve the obtained solid in 6L of ammonia water, wash with dichloromethane (2L×3), stir for 15-20 minutes each time, Separate the liquid, adjust the pH of the aqueous ammonia layer to 8-9 with glacial acetic acid (control the temperature at 0-10°C during the period), stir for 0.5-1 hour, filter, dissolve the obtained solid in 2L of acetonitrile, add 8L of water dropwise, stir for 2-3 hours, and filter , the obtained solid was dissolved in 2L of acetonitrile again, 8L of ...
Embodiment 3
[0047]
[0048] Add N,N-dimethylformamide (DMF) 800ml, sodium trifluoroethoxide 500g, R-2-(((4-chloro-3-methyl-2-pyridyl)methyl) Sulfinyl)benzimidazole 160g, heated to 90-100°C, reacted for 3-4 hours, HPLC detection formula (I) compound content was 0.04%, sulfone impurity content 0.2%; the reaction solution was cooled to room temperature, drop Add 4.8L of 5% sodium chloride aqueous solution, lower to 0-10°C and stir for 0.5-1 hour, filter, dissolve the obtained solid in 4.8L of ammonia water, wash with dichloromethane (1.6L×3), stir for 15-20 Minutes, liquid separation, the ammonia water layer was adjusted to pH 8-9 with glacial acetic acid (temperature control 0-10°C during the period), stirred for 0.5-1 hour, filtered, the obtained solid was dissolved in 1.6L of acetonitrile, 6.4L of water was added dropwise, and stirred for 2- After 3 hours, filter, the obtained solid was dissolved in 1.6L of acetonitrile again, 6.4L of water was added dropwise, stirred for 2-3 hours, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com