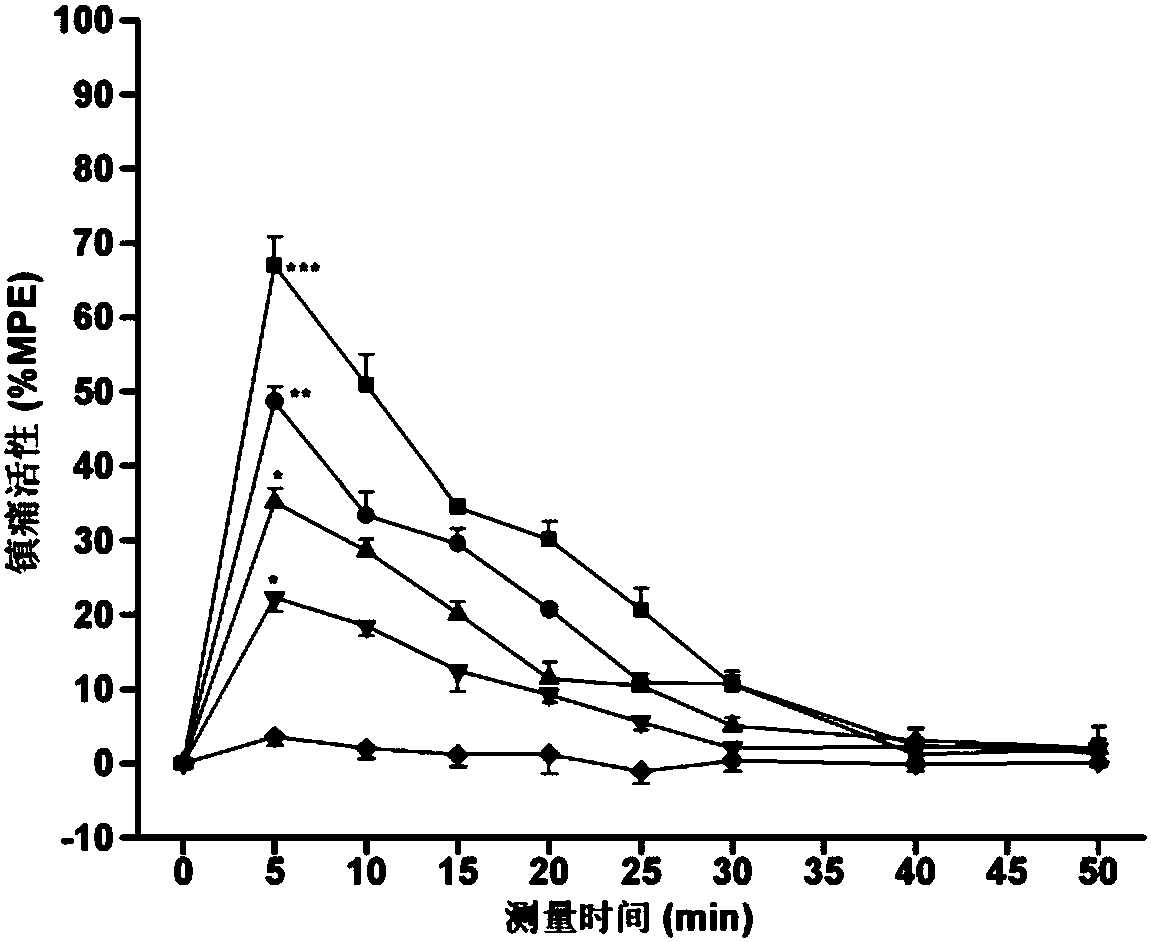
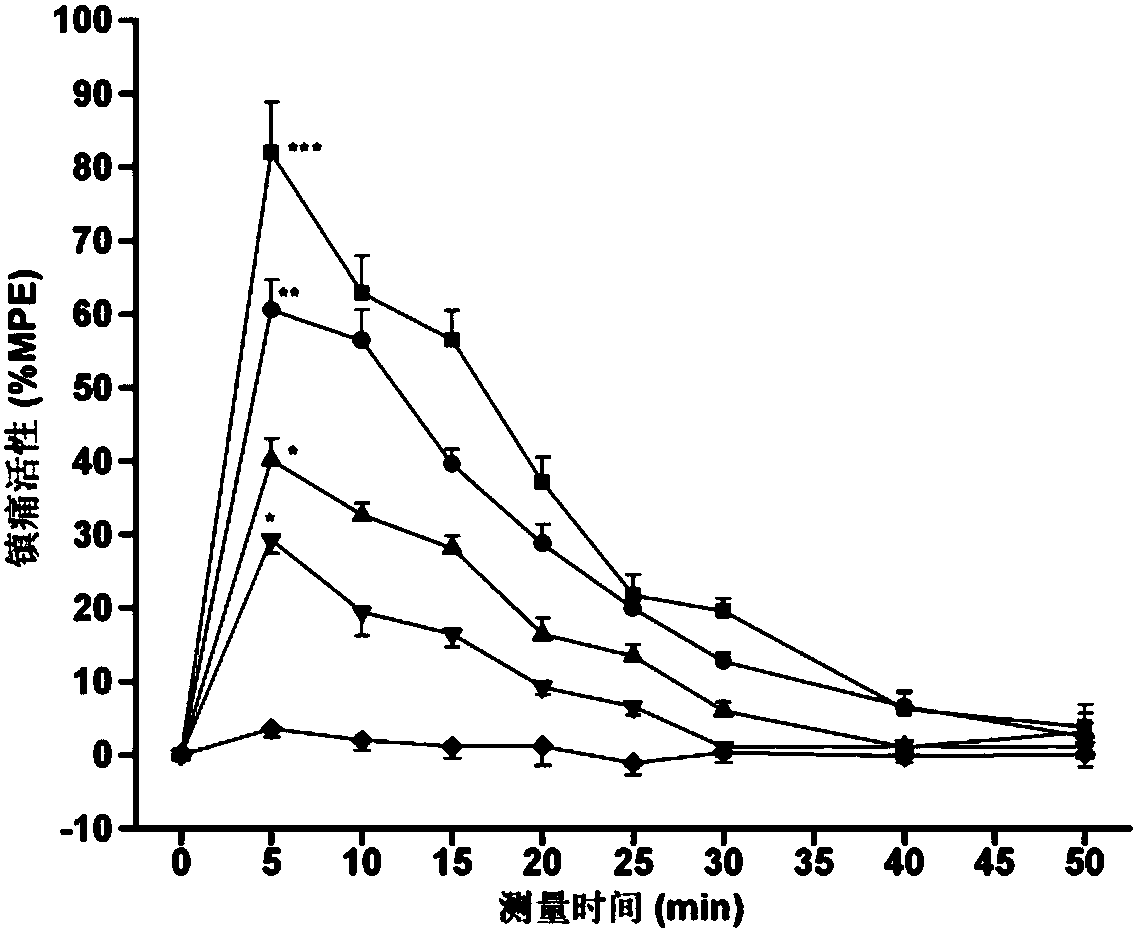
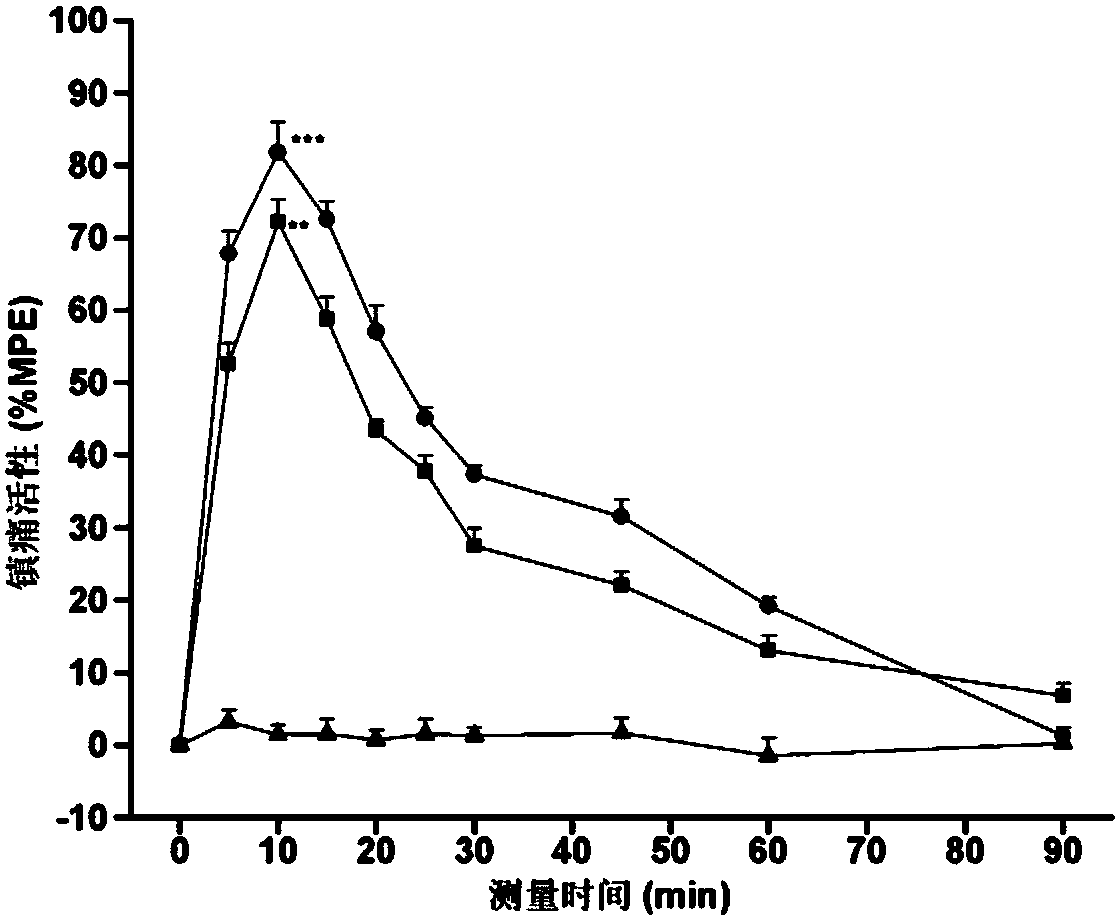
C-terminal aromatic ester modified endomorphin-1 analogue as well as synthetic method and application thereof
A technology of endomorphin and synthesis method, applied in the field of endomorphin-1 analogs and its synthesis, can solve the problem of endomorphin-1 with short analgesic time, poor analgesic effect, analgesic tolerance, cardiovascular and respiratory Systemic side effects and other problems, to achieve the effect of improving enzymatic stability, improving fat solubility, improving utilization rate and analgesic efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0040] Specific embodiment 1: The structure of the endomorphin-1 analog modified by aromatic esterification at the C-terminal of this embodiment is as follows:
[0041]
[0042] where n=1, 2 or 3.
specific Embodiment approach 2
[0043] Specific embodiment 2: The synthesis method of endomorphin-1 analogs modified by C-terminal aromatic esterification in this embodiment includes the following steps:
[0044] 1. Synthesis of benzyloxycarbonyl-tryptophan benzyl ester / phenethyl ester / phenyl propyl ester
[0045] Add benzyloxycarbonyl-tryptophan and 4-dimethylaminopyridine to the redistilled dichloromethane reaction solution, then add aromatic alcohol, and ice-bath at 0-5°C for 5-10 minutes to obtain a reaction mixture; wherein benzyloxycarbonyl -The molar ratio of tryptophan and 4-dimethylaminopyridine is 1:(0.3~0.5), and the volume ratio of benzyloxycarbonyl-tryptophan and aromatic alcohol is 1mol:(1~4)mL; The aromatic alcohol is benzyl alcohol, phenylethyl alcohol or phenylpropanol;
[0046] Dissolve N,N'-dicyclohexylcarbodiimide in redistilled dichloromethane solution, add it to the reaction mixture, stir and react at 0-5°C for 20-40min, then react at room temperature for 10-15h; The molar ratio of ox...
specific Embodiment approach 3
[0060] Specific embodiment three: this embodiment differs from specific embodiment two in that: the specific method of washing in step one is: washing with 5% citric acid solution, saturated sodium bicarbonate solution and saturated sodium chloride solution successively. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com