Tumor specificity gamma delta T cell preparation method
A tumor-specific, tumor cell-based technology, applied in the field of preparation of tumor-specific γδT cells, can solve problems such as complex steps, uncontrollable conditions, and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention provides a method for preparing tumor-specific γδT cells, comprising the following steps:
[0051] S1, extracting autophagosomes derived from tumor cells;
[0052] As mentioned above, in the present invention, the selected tumor cells are prostate cancer cells.
[0053] As mentioned above, it needs to be understood that the autophagosome is formed by a small part of the cytoplasm surrounded by a double membrane, and the digested substances are various components contained in the cytoplasm, such as mitochondria, fragments of the endoplasmic reticulum, etc. It is one of the components of the cell vacuole system by fusing with lysosomes to degrade their contents. Also known as autolysosomes. Under pathological atrophy, autophagosomes increase and lipofuscin granules appear in the cytoplasm of atrophic cells such as cardiomyocytes and hepatocytes.
[0054] S2, collecting peripheral venous blood and obtaining mononuclear cells therefrom;
[0055] As mention...
Embodiment
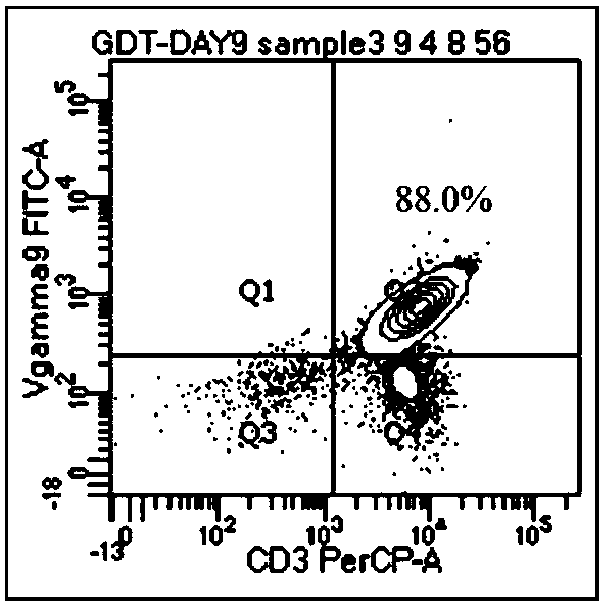
[0096] The present invention provides a method for preparing tumor-specific γδT cells. Prostate cancer DU145 cells are cultured, cells are induced by factors to produce autophagosomes, and they are extracted and identified, including the following steps:
[0097]1. For the cultivation of prostate cancer cells, cultivate them with MEM medium containing 10% FBS+100U / mL penicillin-streptomycin. The area of -90% shall prevail), add 100nM rapamycin (autophagy inducer), 100nM bortezomib (proteasome inhibitor) and 10nM NH4Cl (lysosomal chemoattractant, prevent lysosome and autophagy After 18-24h treatment, the cells were collected.
[0098] 2. Pour the cell culture supernatant into a 50mL centrifuge tube, centrifuge at 1600r / min for 10min. The adherent cells were digested with trypsin and centrifuged at 1000r / min for 10min. After centrifugation, the pelleted cells were resuspended in saline and centrifuged after repeated pipetting. The supernatant obtained by centrifugation was t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com