Recombinant hansenula polymorpha for expressing Zika virus structural protein E and construction method thereof
A technology of Hansenula polymorpha and Zika virus, applied in the direction of microorganism-based methods, viruses, viral peptides, etc., can solve the problems of non-expression and low efficiency of ZKE protein expression, and achieve low cost, good immunogenicity, Cultivate Simple Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] The present invention will be described in further detail below in conjunction with the accompanying drawings and embodiments.
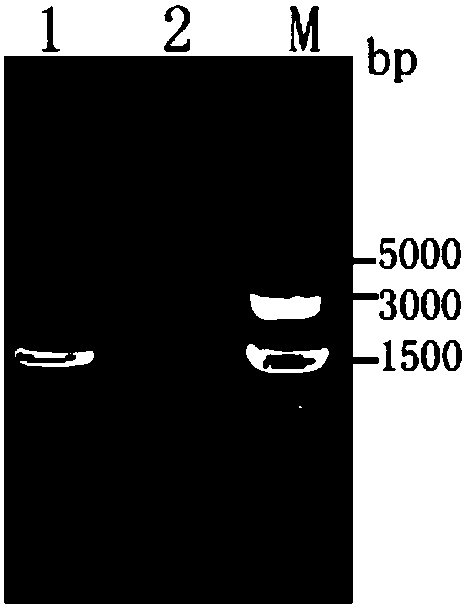
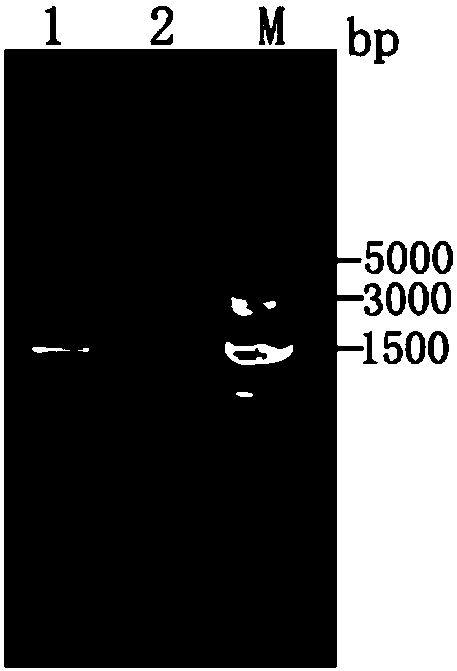
[0022] (1) Construction of pHMOXG-alpha-ZKE recombinant expression vector containing Zika virus structural protein E gene fragment 1. According to the reported Zika virus structural protein E gene information (GenBank: KU820899.2), artificially design Zika virus to be expressed Viral structural protein E gene fragment (designed in May 2017), the two ends of which can be added with amplification primers (primer 1 and primer 2, designed in July 2017) EcoRI (carbon terminus, see sequence underlined) position) and restriction site NotI (nitrogen terminus, see sequence italicized position). The artificially designed Zika virus structural protein E gene fragment sequence is shown in SEQ.ID.NO.1, which is an artificially synthesized sequence.
[0023] 2. After the plasmid (pMD18-T, Takara) containing the designed Zika virus structural protein E gene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com