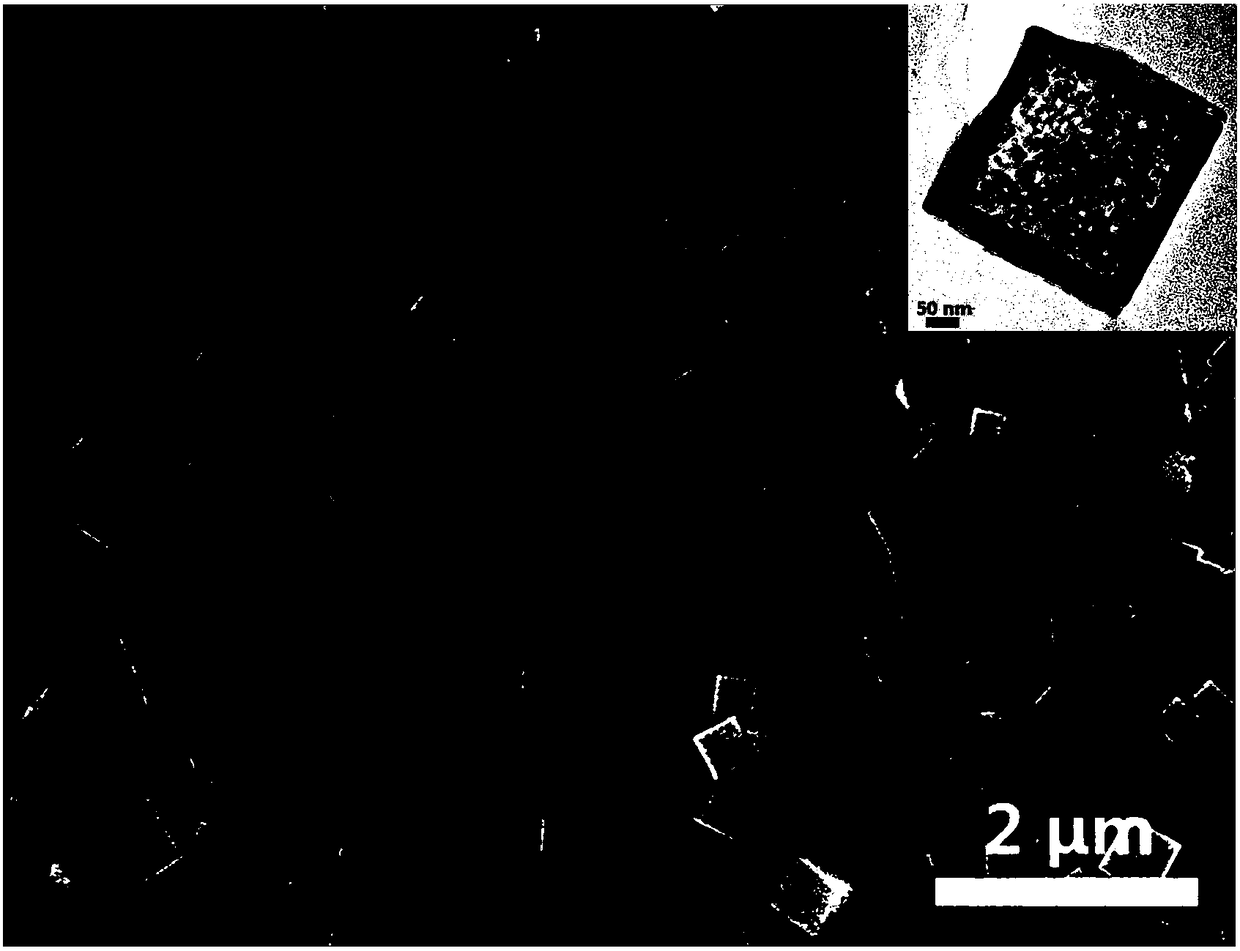
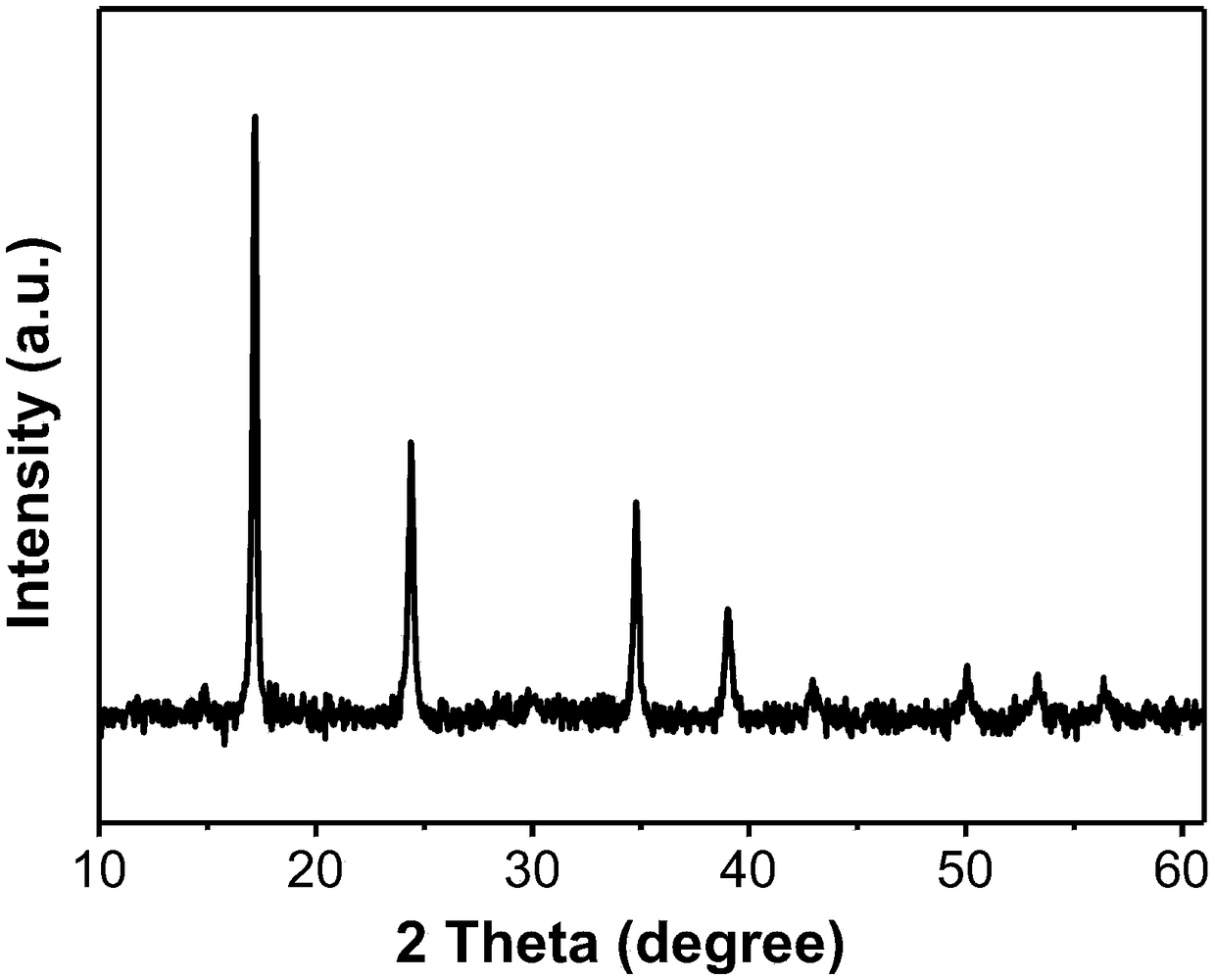
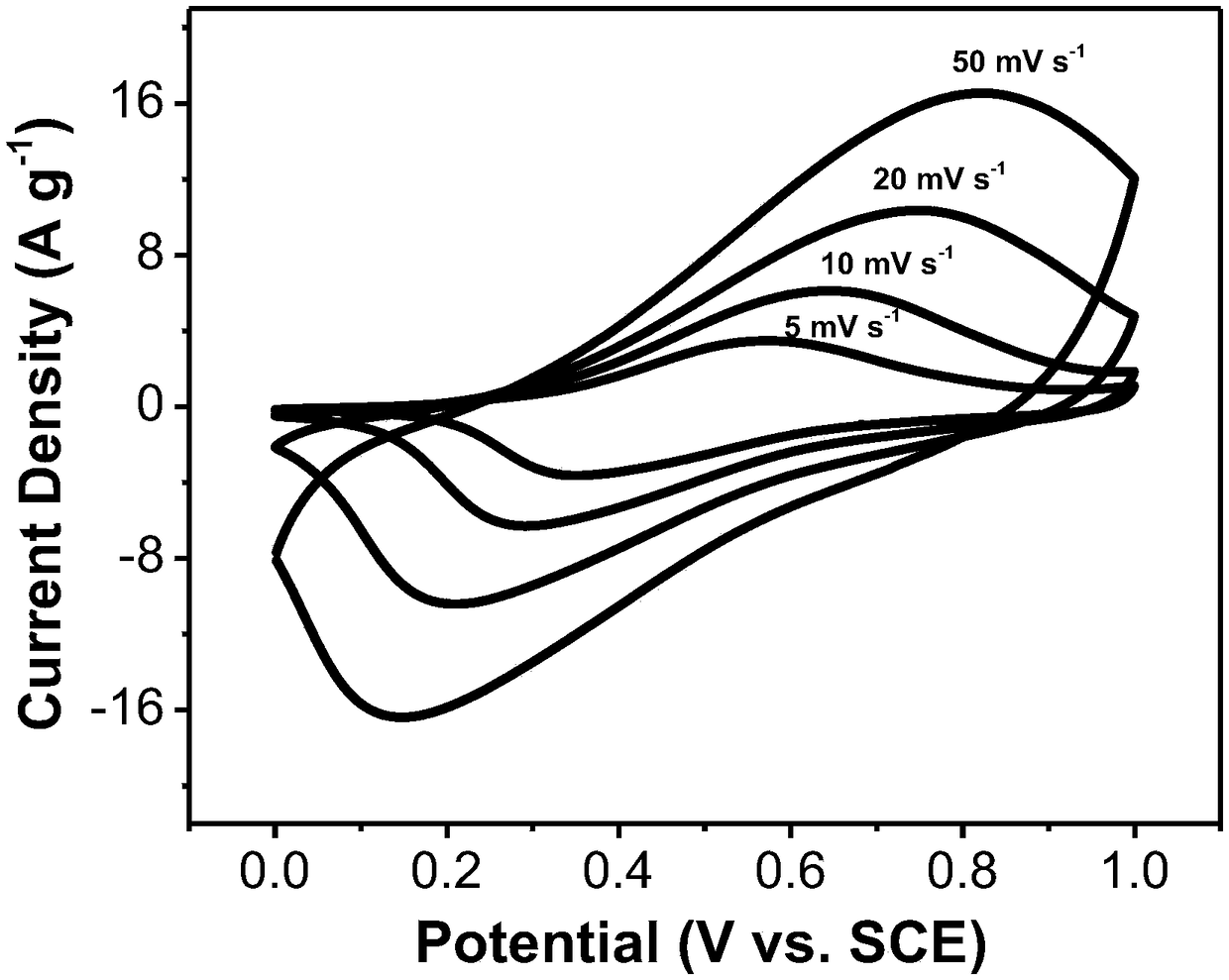
Preparation method of nano hollow structure prussian blue and analogues thereof
A technology of hollow structure and Prussian blue, which is applied in the field of preparation of nano-hollow structure Prussian blue and its analogues, can solve problems such as damage to health, and achieve the effect of cheap raw materials, uniform appearance and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The steps of the preparation method of the present invention are as follows:
[0035] Step 1: Dissolve the manganese salt evenly in the polymer aqueous solution, add an appropriate amount of potassium ferricyanide or sodium ferricyanide aqueous solution and stir at room temperature to form a manganese ferricyanide suspension; the polymer in the polymer aqueous solution in the present invention The substance can generate a certain force with manganese ions, and increase the viscosity of the solution, thereby inhibiting the generation rate of manganese ferricyanide, which is beneficial to the formation of cubic morphology.
[0036] The preferred molar ratio of manganese salt to potassium ferricyanide or sodium ferricyanide is controlled between 3:2-4:2. If there are too few manganese salts, excess potassium ferricyanide or sodium ferricyanide will react with the metal salt ions added subsequently to generate granular ferricyanides that are difficult to remove, which will ...
Embodiment 1
[0045] 1. Preparation of manganese ferricyanide template suspension
[0046] Dissolve 0.35g of polyvinylpyrrolidone in 10ml of absolute ethanol to form solution A; weigh 100mg of MnSO 4 ·H 2 O powder was uniformly dissolved in 10ml deionized water to form solution B. Slowly add solution B to solution A and continue stirring for 30 min, then add 10 ml of 0.03M potassium ferricyanide aqueous solution dropwise. After continuous stirring at room temperature for 12 hours, a suspension of manganese ferricyanide can be obtained.
[0047] 2. Preparation of nano hollow cobalt ferricyanide
[0048] 10ml 0.02M Co(NO 3 ) 2 The aqueous solution was added dropwise to the above suspension, stirred rapidly for 5 minutes, and then stood at room temperature for 10 hours to allow sufficient cation exchange reaction. Afterwards, centrifuge with deionized water at a rate of 6000r / min, and dry the obtained precipitate in an oven at 80°C for 18 hours to obtain a nano-hollow cobalt ferricyanide...
Embodiment 2
[0050] 1. Preparation of manganese ferricyanide template suspension
[0051] Dissolve 0.35g of polyvinylpyrrolidone in 10ml of absolute ethanol to form solution A; weigh 100mg of MnSO 4 ·H 2 O powder was uniformly dissolved in 10ml deionized water to form solution B. Slowly add solution B to solution A and continue stirring for 30 min, then add 10 ml of 0.03M potassium ferricyanide aqueous solution dropwise. After continuous stirring at room temperature for 15 hours, a suspension of manganese ferricyanide can be obtained.
[0052] 2. Preparation of nano hollow copper ferricyanide
[0053] 10ml of 0.02M Cu(NO 3 ) 2 The aqueous solution was added dropwise to the above suspension, stirred rapidly for 8 minutes and then left at room temperature for 8 hours to allow sufficient cation exchange reaction. Afterwards, centrifuge with deionized water at a rate of 6000 r / min, and dry the obtained precipitate in an oven at 80°C for 18 hours to obtain a hollow nano-copper ferricyanid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com