Large-scale preparation technology of endotoxin-free plasmid
A technology of endotoxin and quality, applied in the biological field, can solve problems such as inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Small-scale preparation of ten grams or less of endotoxin-free lentiviral plasmid
[0025] Prepare lentivirus purification plasmids with a scale of 10 grams or less. Shake flasks are selected for cell fermentation, and the cells are collected by a centrifuge. The clarified lysate after lysis is centrifuged in a refrigerated high-speed centrifuge, and 1ml or 5ml of anion exchange is used for column chromatography. Prepacked columns.
[0026] 1. Shake flask fermentation
[0027] Take a recombinant strain from the seed bank, streak it on the resistance plate, and expand the single colony in 5mL liquid medium containing the corresponding antibiotic for 8h, and then inoculate it into a 1L container with a ratio of 1:1000. In a 3L shake flask of the culture medium, shake culture at 180-220rpm at 37°C for 16h.
[0028] 2. Cell collection
[0029] Refrigerated high-speed centrifuge at 8000g 4°C for 3min to collect the bacterial cells, and weigh the wet weight of t...
Embodiment 2
[0037] Example 2: Preparation of endotoxin-free lentiviral plasmids on a scale of more than ten grams
[0038] For the preparation of purified lentivirus plasmids of more than 10 grams, shake flask fermentation is limited by its yield, so it is necessary to use a fermenter for fermentation. The fermenter can increase the bacterial cell production per unit medium on a large scale, and can be scaled up in parallel; For clarification, you can choose a refrigerated high-speed centrifuge, which is time-consuming, or you can choose TFF. Compared with the two, TFF is more suitable for large-scale production, but the cost of consumables is higher; the selection of column volume can refer to the binding amount of 2g / ml. choose.
[0039] 1. Fermentation tank fermentation bacteria
[0040] Take a recombinant strain from the seed bank, streak it on the resistance plate, expand the single colony in the liquid medium containing the corresponding antibiotic, and then inoculate it into a 100...
Embodiment 3
[0050] Embodiment 3: Culture medium is optimized to improve plasmid yield
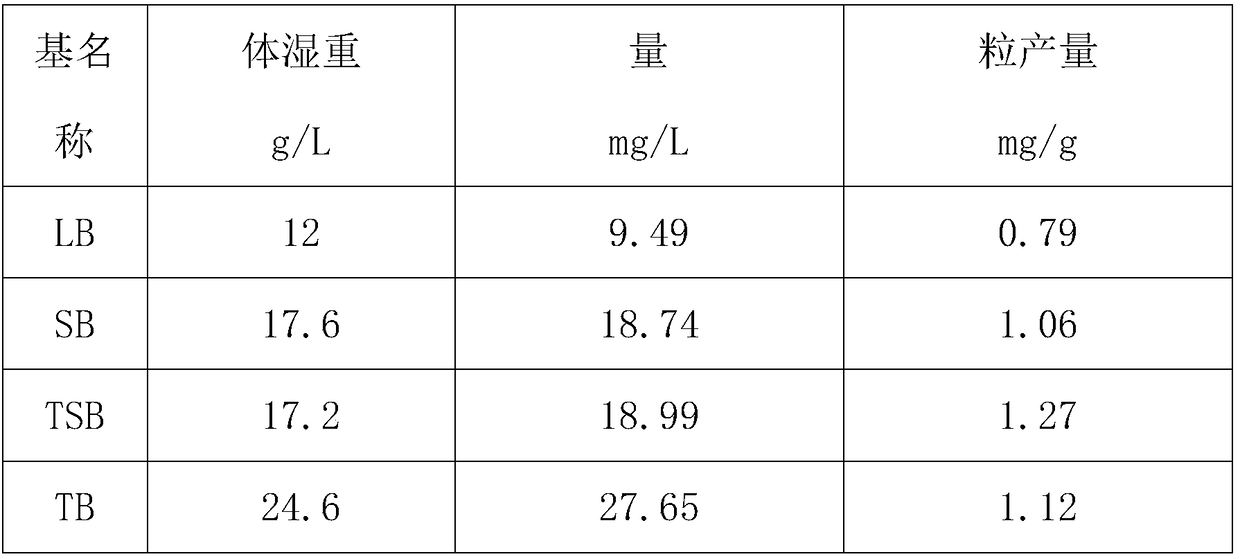
[0051] Comparing LB, SB, TSB, TB four kinds of media under the same culture conditions, the wet weight of bacteria and plasmid yield per unit volume of culture medium, the medium with high plasmid yield was selected out of them. Shake the bacteria in the flask under the same conditions for the following four culture media, take 25ml of the bacterial liquid each, and extract the plasmid with an endotoxin-free plasmid extraction kit.
[0052] LB medium formula: 1% (W / V) tryptone, 0.5% (W / V) yeast extract, 1% (W / V) NaCl.
[0053] SB medium formula: 3.5% (W / V) tryptone, 2.0% (W / V) yeast extract, 0.5% (W / V) NaCl, 0.5% (V / V) 1M NaOH.
[0054] TSB medium formula: 1.5% (W / V) tryptone, 0.5% (W / V) soybean peptone, 0.5% (W / V) NaCl, adjust the pH to 7.2±0.2.
[0055] TB medium formula: 1.2% (W / V) tryptone, 2.4% (W / V) yeast extract, 0.4% (V / V) glycerol, 17mM KH2PO4, 72mM K2HPO4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com