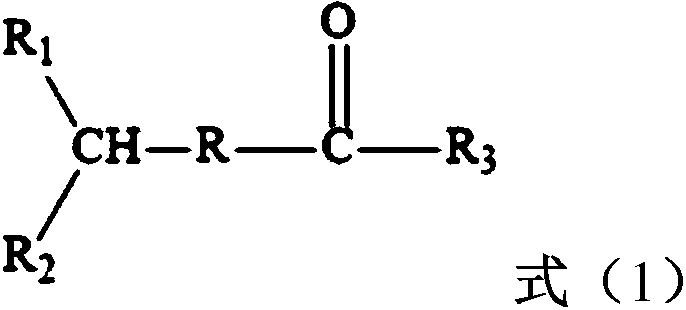
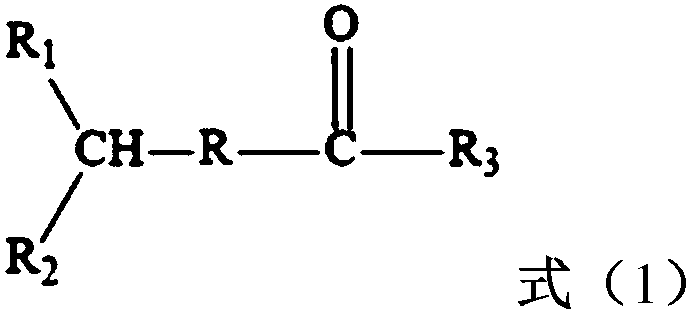
H-shaped polymer and preparation method thereof
A technology of polymers and prepolymers, applied in the field of polymers, can solve problems such as low molecular weight of arm chains and poor controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The method for preparing H-type polymer provided by the invention comprises the following steps:
[0013] (1) In the presence of a dianion initiator and a solvent, the first monomer is subjected to a two-way anionic polymerization reaction, and then an epoxy compound is used to carry out a capping reaction, and then a dihaloacyl halide is added to carry out an acylation reaction to obtain a prepolymerized thing;
[0014] (2) In the presence of a catalyst and a complexing agent, the prepolymer initiates a living free radical polymerization reaction of the second monomer.
[0015] The present invention is not particularly limited to the kind of described dianion initiator, can be the initiator of various existing bidirectional anionic polymerization that can initiate, and its example includes but not limited to: Alkali metal-naphthalene initiator, organic dilithium At least one of initiators and the like. Wherein, the alkali metal-naphthalene initiator may be at least o...
Embodiment 1
[0034] This example is used to illustrate the H-type polymer provided by the present invention and its preparation method.
[0035] Under a nitrogen atmosphere and anhydrous conditions, 1200g of benzene, 1200g of tetrahydrofuran and 120.0g (1.152mol) of styrene were added to the reactor, and then 0.6mmol of naphthalene lithium was added to initiate polymerization. After polymerization at 20°C for 2 hours, 0.1322g ( 3mmol) ethylene oxide and reacted at 20°C for 1h, then added 1.0107g (3.6mmol) 2,2-dibromoacetyl bromide and reacted at 20°C for 6h, followed by precipitation, filtration and drying to obtain 2 , 2-dibromoacetoxy terminated prepolymer, its number average molecular weight Mn=213893 and molecular weight distribution MWD=1.13 were measured by GPC.
[0036] 10.0g of the above-mentioned prepolymer was added to 500g of tetrahydrofuran, and 0.0537g of bromine was added in the ratio of bromine atom in the prepolymer: cuprous bromide: 2,2'-bipyridine molar ratio of 1:2:4 Cu...
Embodiment 2
[0039] This example is used to illustrate the H-type polymer provided by the present invention and its preparation method.
[0040] Under nitrogen atmosphere and anhydrous conditions, add 2400g toluene and 240.0g (2.305mol) styrene into the reactor, then add 2.3mmol sodium naphthalene to initiate polymerization, after polymerization at 30°C for 1h, add 0.2643g (6mmol) cyclo Oxyethane and reacted at 30°C for 0.5h, then added 2.5828g (9.2mmol) 2,2-dibromoacetyl bromide and reacted at 40°C for 1h, followed by precipitation, filtration and drying to obtain 2,2 -The prepolymer at the end of dibromoacetoxy group, the number average molecular weight Mn=107485 and the molecular weight distribution MWD=1.09 as measured by GPC.
[0041] 5.0g of the above-mentioned prepolymer was added to 500g of tetrahydrofuran, and 0.0801g of bromine was added in the ratio of bromine atom in the prepolymer: cuprous bromide: 1,10-phenanthroline molar ratio of 1:3:6 Cuprous chloride and 0.2012g of 1,10-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com