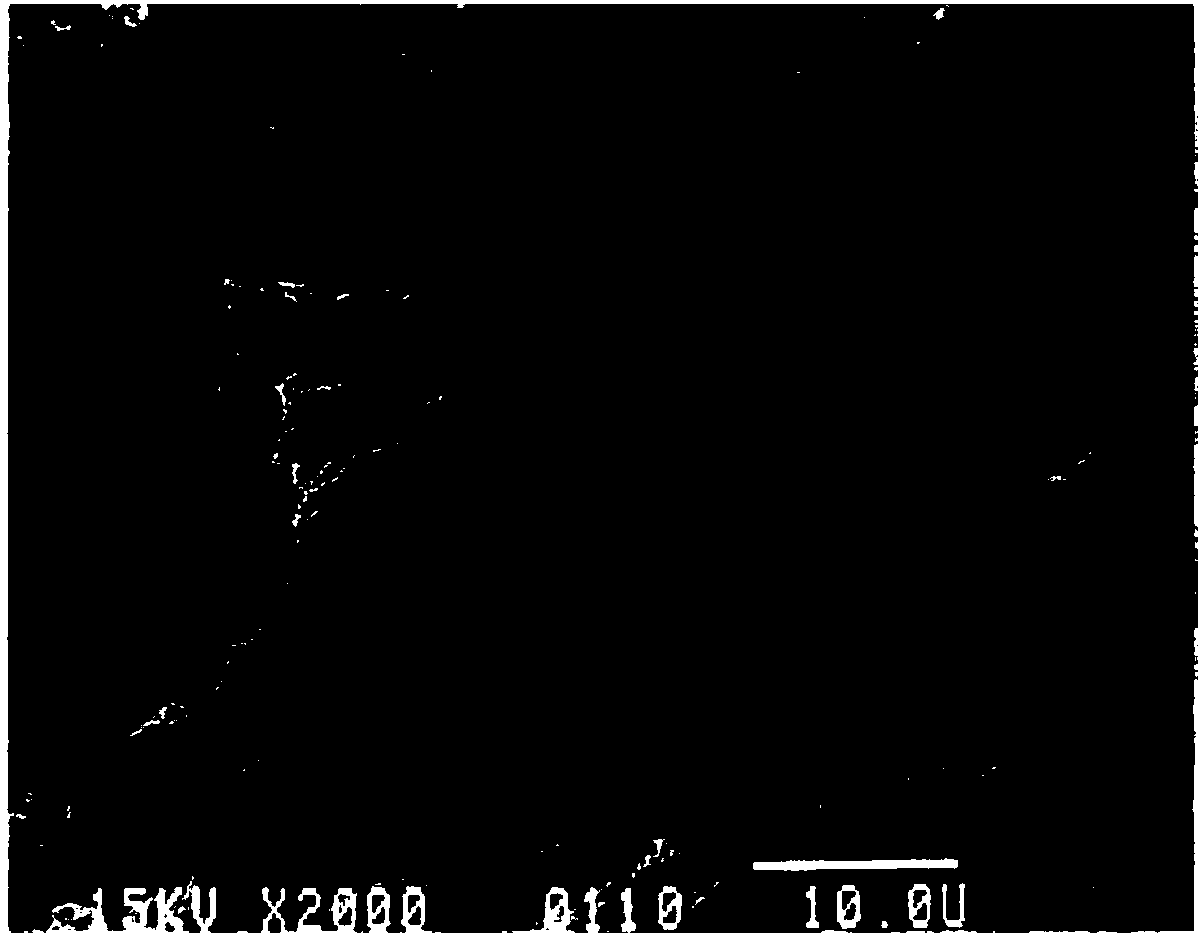Serotype 8 recombinant adeno-associated virus preparation method
A technology of recombinant adenovirus and serum, which is applied in the field of preparation of recombinant adeno-associated virus, can solve the problems of long time consumption, small flux, and reducing the immune response of the body, and achieve the goal of increasing virus titer, large specific surface area, and large virus output Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The present invention provides a preparation method of recombinant adeno-associated virus of serotype 8, comprising the following steps:
[0037] (1) 293T cells were cultured in a sheet carrier using serum-free medium.
[0038] 293T cells were inoculated on the sterilized and soaked sheet carrier, and the cells were cultured at a temperature of 37°C, a pH of 6.8-7.6, a DO of 30%-60%, and a stirring speed of 60 rpm. The amount of cells on the sheet carrier reaches 1×10 8 ~3×10 8 cells / g. Animal cells are inoculated on the sheet-like carrier, under the controlled culture conditions of the bioreactor, the nutrient consumption is detected, and the liquid is replenished or changed, and the cultured cells reach 1×10 8 ~3×10 8 cells / g, the cells on the sheet carrier form a healthy and dense monolayer, which is conducive to the transfection of the plasmid. Excessively high cell density needs to consume a large amount of medium, and the DNA-transfection complex cannot be use...
Embodiment 1
[0053] Taking a 500ml basket bioreactor as an example, the culture medium adopts 293Free style serum-free medium produced by Life Company to produce and prepare serotype 8 recombinant adeno-associated virus.
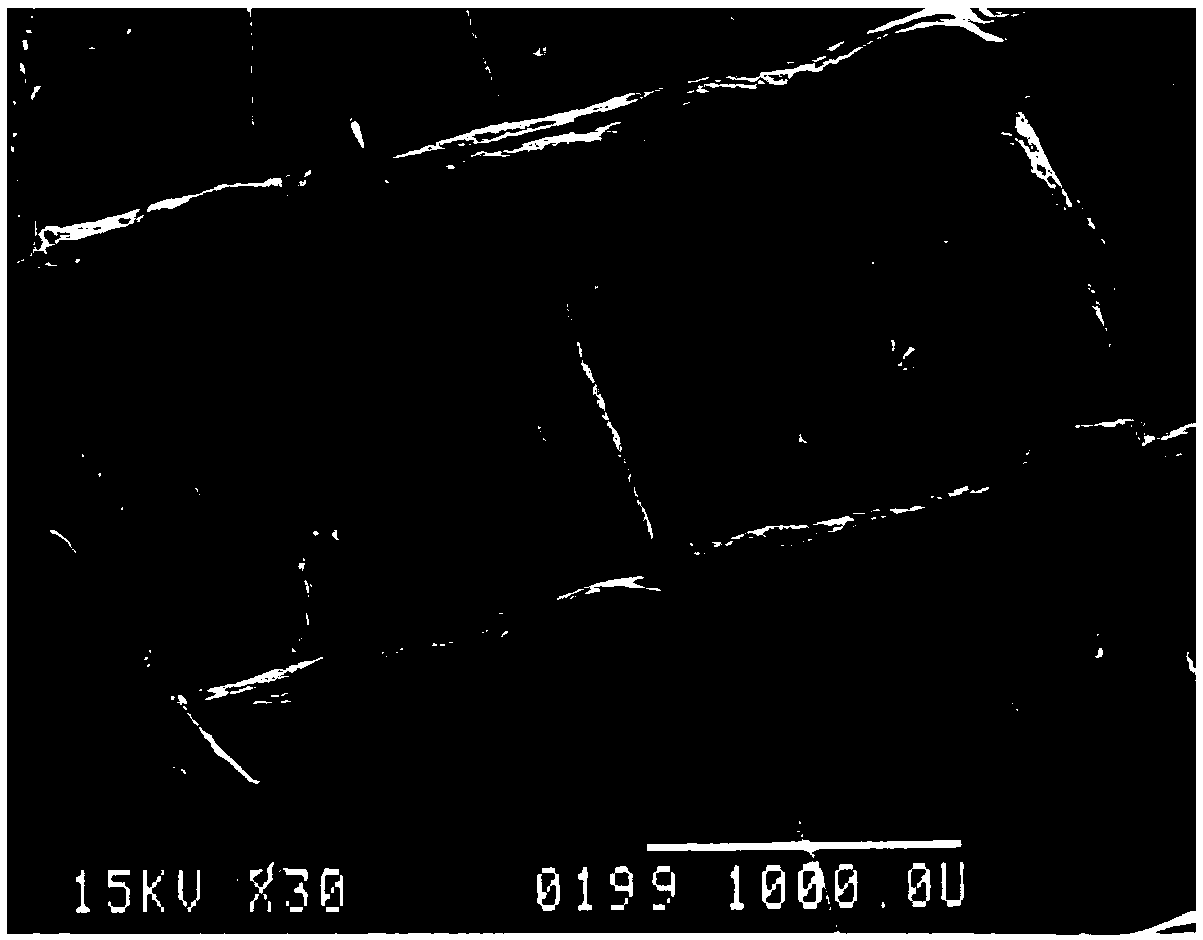
[0054] The amount of the sheet carrier is about 10g, made of polyester fiber, round, 1cm in diameter, figure 1 is the appearance picture of the sheet carrier used in the present invention; figure 2 It is the surface morphology and structure shown by the high-resolution pictures of the sheet carrier used in the present invention. First, soak it with PBS, then rinse it with PBS, then soak it with culture medium overnight, then soak it with PBS and sterilize it by autoclaving, and remove it before use. All PBS.
[0055] Inoculate 5 × 10 on the sheet carrier in the bioreactor 7 A 293T cell, DO is controlled at 30-60%, pH value is maintained at 7.0-7.2, cultured and grown at 37°C for 5-7 days, and glucose digestion is detected during the stirring paddle speed of 60rpm / min,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com