Huperzine A slow-release orally disintegrating tablets and preparation method thereof
A technology of huperzine A and orally disintegrating tablets, which is applied in the fields of pharmaceutical formulation, pill delivery, drug delivery, etc., can solve problems such as difficulty in swallowing and poor patient compliance, and achieve the goal of solving inconvenience in swallowing, simple preparation method, and avoiding toxic and side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The huperzine A sustained-release microspheres provided in this example are prepared by the following method:
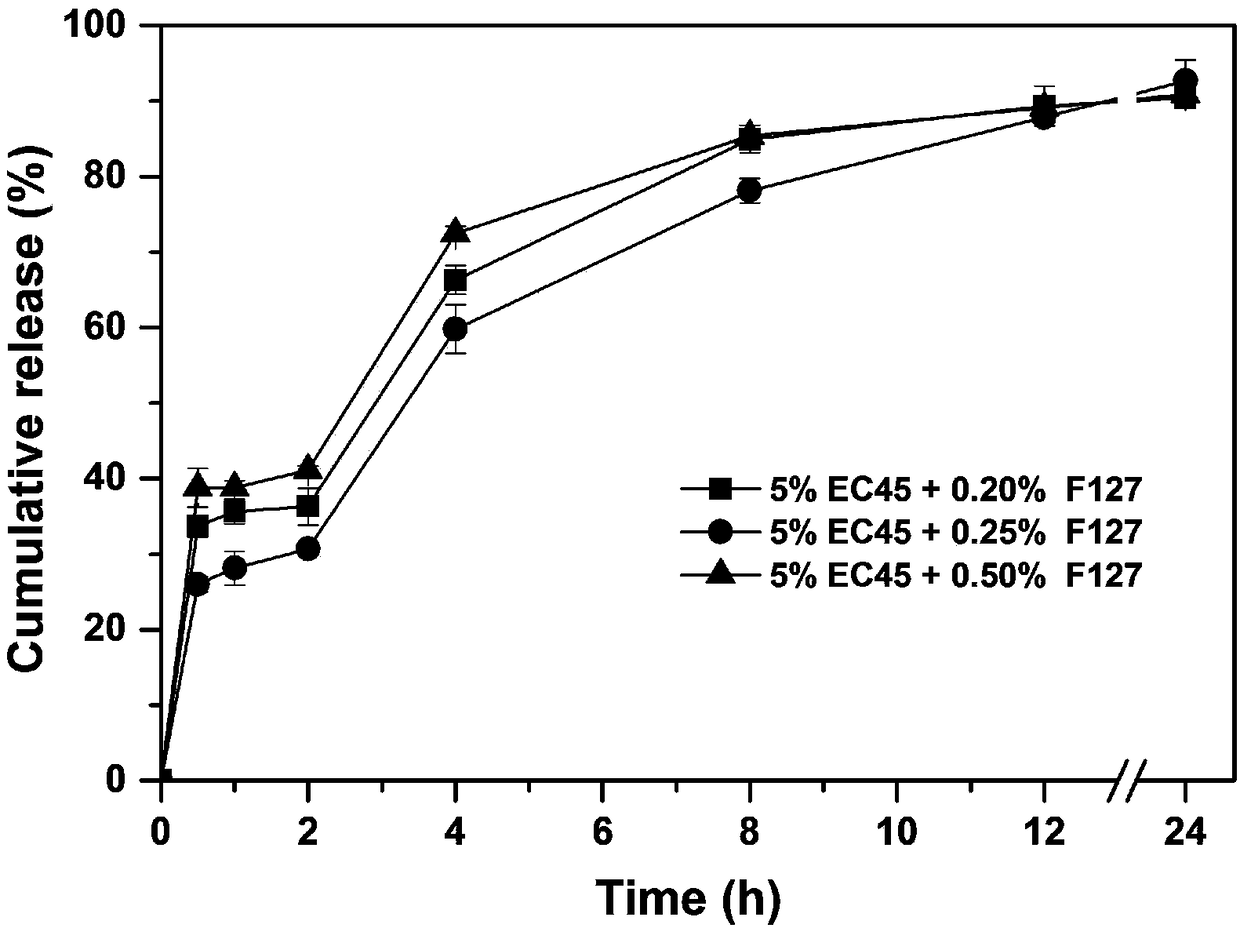
[0043] According to the composition of the prescription shown in Table 1, a certain amount of huperzine A (theoretical drug loading accounts for 0.4% of the dry weight of the skeleton material), the skeleton material ethylcellulose (EC45) and poloxamer (F127), were dissolved in In 200ml of 80% v / v ethanol aqueous solution, the drug solution was prepared; wherein the concentration of the skeleton material ethyl cellulose was 5% w / v, and the concentrations of the skeleton material poloxamer 127 were 0.20% w / v, 0.25% w / v, respectively. %w / v, 0.5%w / v.
[0044] The obtained drug solution was uniformly fed into UPPS to prepare huperzine A sustained-release microspheres, and three kinds of huperzine A sustained-release microspheres with different F127 contents were obtained. The instrument parameters used are: liquid supply rate 8ml / min, rotating disc rotation speed 9...
Embodiment 2
[0048] The huperzine A sustained-release microspheres provided in this example are prepared by the following method:
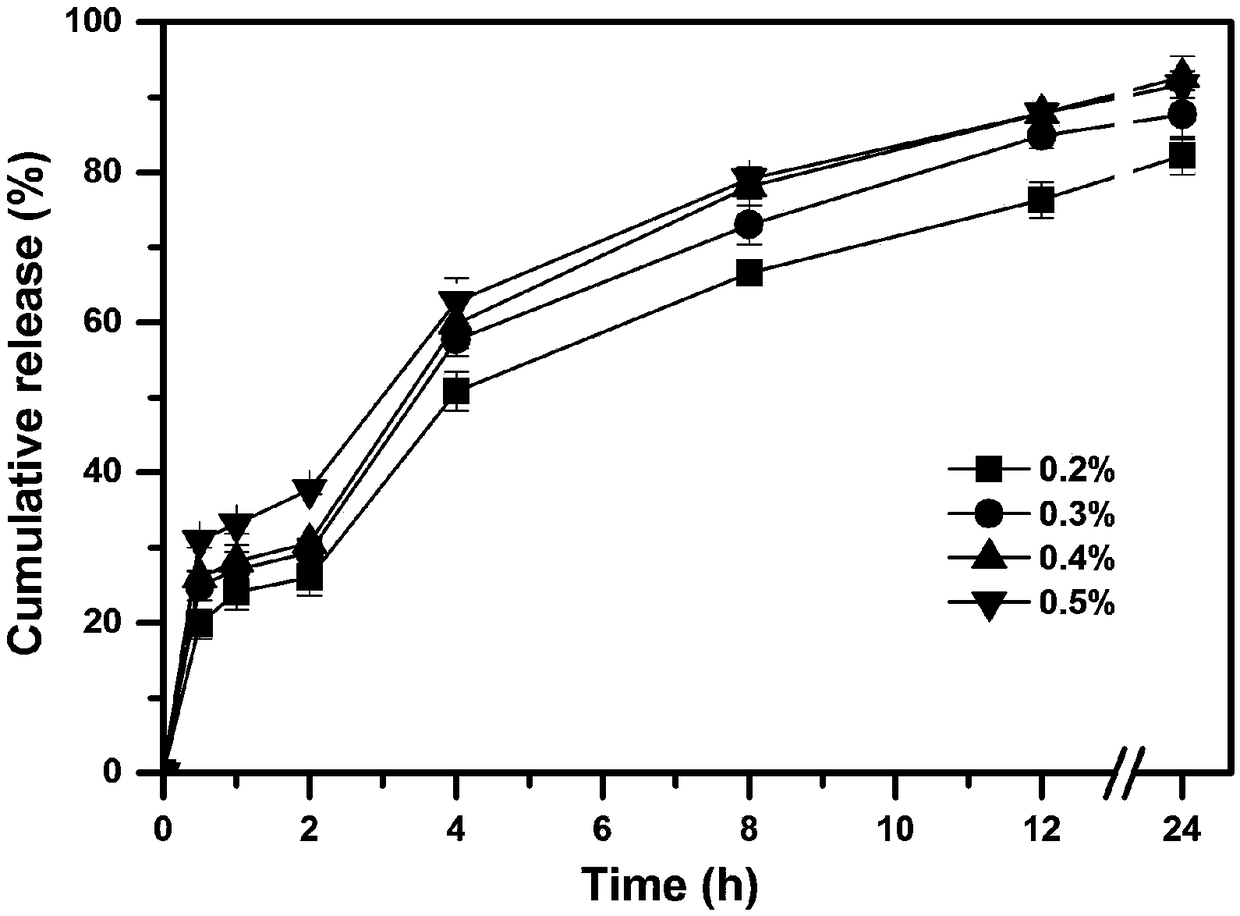
[0049] According to the prescription composition shown in table 2, a certain amount of huperzine A (theoretical drug loading accounts for 0.2wt%, 0.3wt%, 0.4wt%, 0.5wt% of the dry weight of the skeleton material respectively), the skeleton material ethyl fiber (EC45) and poloxamer 127 (F127), dissolved in 100ml of 80% v / v ethanol aqueous solution, to prepare a drug solution; wherein the concentration of the skeleton material ethylcellulose is 5% w / v, the skeleton material poloxamer The concentration of Loxamer 127 was 0.25% w / v.
[0050] The obtained drug solution was uniformly fed into UPPS to prepare huperzine A sustained-release microspheres, and four kinds of huperzine A sustained-release microspheres with different drug loadings were obtained. Wherein the instrument parameter used is identical with embodiment 1.
[0051] Table 2 contains the composition...
Embodiment 3
[0054] The huperzine A sustained-release microspheres provided in this example are prepared by the following method:
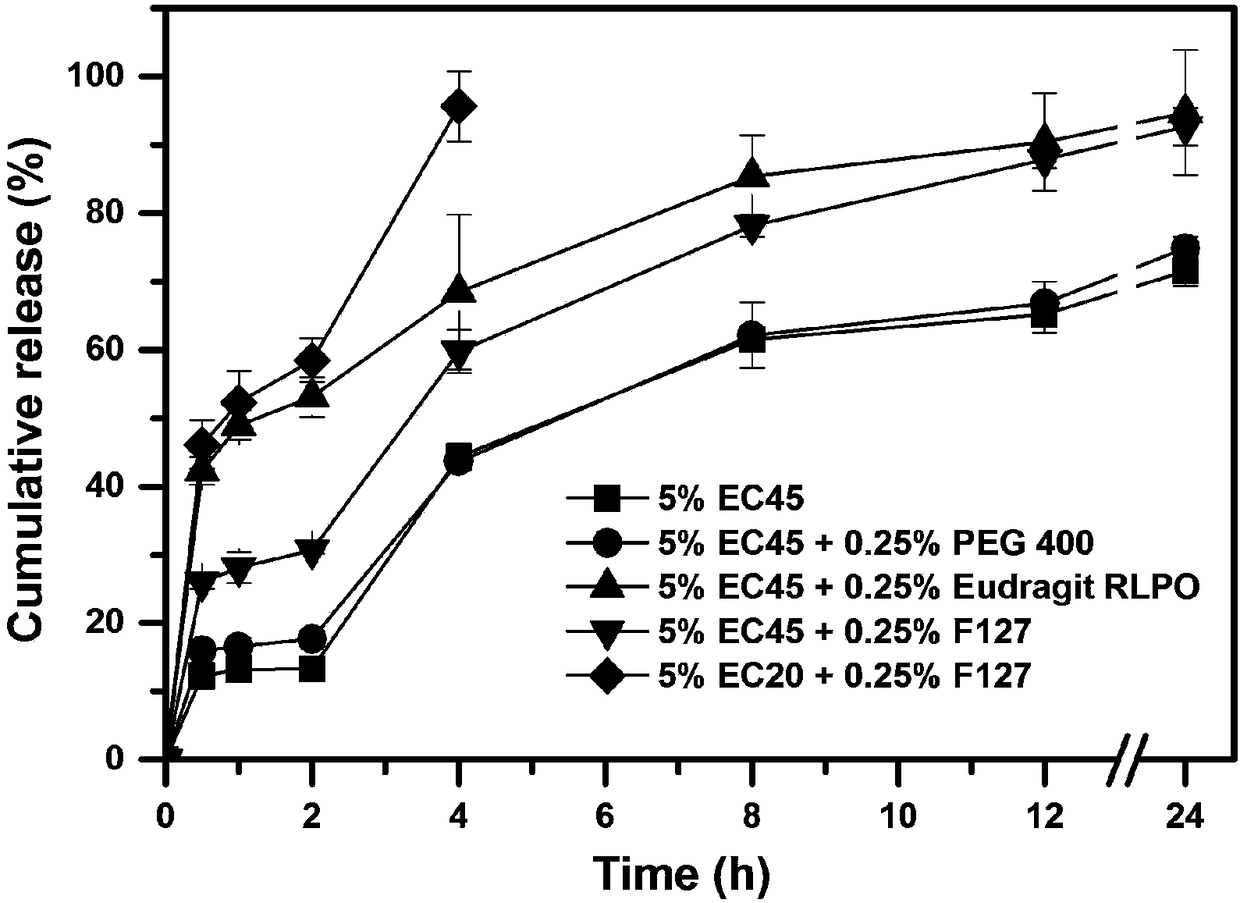
[0055] According to the composition of the prescription shown in Table 3, a certain amount of huperzine A (theoretical drug loading accounts for 0.4wt% of the dry weight of the framework material) and the framework material are dissolved in 100ml of 80% v / v ethanol aqueous solution to prepare Drug solution; wherein the concentration of framework material A is 5% w / v, and the concentration of framework material B is 0.25% w / v.
[0056] The obtained drug solution was uniformly fed into UPPS to prepare huperzine A sustained-release microspheres, and four kinds of huperzine A sustained-release microspheres with different compositions were obtained. Wherein the instrument parameter used is identical with embodiment 1.
[0057] The prescription composition of table 3 huperzine A sustained-release microspheres
[0058] prescription
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com