Application of silibinin in preparation of drugs for preventing and treating intestinal cancer correlated with colitis
A technology for silibinin and colitis, which is applied to the application field of silibinin in the preparation of drugs for preventing and treating colitis-related bowel cancer, can solve the problems of unsatisfactory curative effect and safety, and achieves reduction of inflammation recurrence and improvement of survival. quality, the effect of inhibiting cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The azoxymethane (AOM) / dextran sodium sulfate (DSS) mouse model is a mouse model of inflammatory bowel disease (inflammatory bowel disease) based on the synergistic effect of the carcinogen AOM and the inflammatory agent DSS. disease, IBD) has gradually developed into a research model of inflammation-related cancer of intestinal cancer.
[0026] Animal feeding methods are as follows:
[0027] AOM dissolved in physiological saline: In the first week of the experiment, according to the weight of each mouse, 10 mg / kg intraperitoneal injection;
[0028] Dissolve DSS in drinking water to a concentration of 2%: 10g DSS+500ml filtered autoclaved water;
[0029] SB dissolved in 0.5% hydroxymethylcellulose (CMC): 750mg / kg orally.
[0030] C57 6w Female mice (purchased from Beijing Animal Research Center)
[0031]
[0032] 2% DSS × 5 days, a total of 3 cycles
[0033] SB 5 days a week x 10 weeks
[0034] Record weight, diet and drinking water every week
[0035] All int...
Embodiment 2
[0038] MTT proliferation assay:
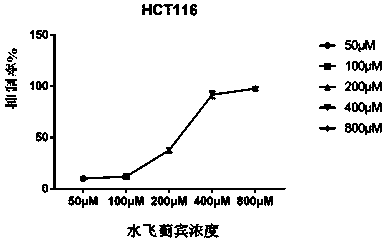
[0039] Cells with good growth status (HCT116 cell line, IMCE cell line) [HCT116 cell line was purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai), and the mouse colonic pre-tumor cell line (Immorto-Min colonic epithelial cell line, IMCE) was purchased from Vanderbilt University, USA introduced], seeded in 96-well plate (5×10 3 each well) for 24 hours, given different concentrations of silibinin (50-800μ / M) to stimulate and continue to culture for 72 hours, and added 10 μL 5 mg / mL MTT [3-(4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide] 4 h, discard the culture medium, add 150 μl DMSO solution to each well, shake for 10 minutes to fully dissolve the crystals, and measure the OD value in a microplate reader at a wavelength of 570 nm. The experimental results showed that silibinin could well inhibit IMCE cells and HCT116 cells. (see image 3 , 4 )
Embodiment 3
[0041] Preparation of paraffin sections and evaluation of histological damage
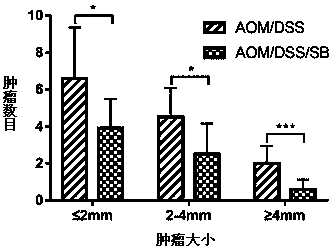
[0042] The colon tissue specimens of each group were made into paraffin sections after being dehydrated, transparent, soaked in wax, embedded, sliced and baked. After the sections are dewaxed and hydrated, hematoxylin staining, hydrochloric acid alcohol color separation, light ammonia water inversion blue and eosin staining are carried out in sequence, and then dehydration, transparency and sealing can be observed under the microscope and histological scoring (inflammation and tumor scores), the results showed that the silibinin intervention group significantly decreased the inflammation score and tumor score, and it was statistically significant (* p 0.001 , AOM / DSS vs AOM / DSS / SB). (see Figure 5 )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com