Application of recombinant human insulin-like growth factor 1 (rhIGF1) in preparation of medicines for treating fragile X syndrome
A syndrome, IGFR1-AKT-S6K technology, applied in the field of medicine, can solve the problems of mouse projection neuron cell body volume, dendritic length, dendritic branching and synaptic density abnormalities, brain volume reduction and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
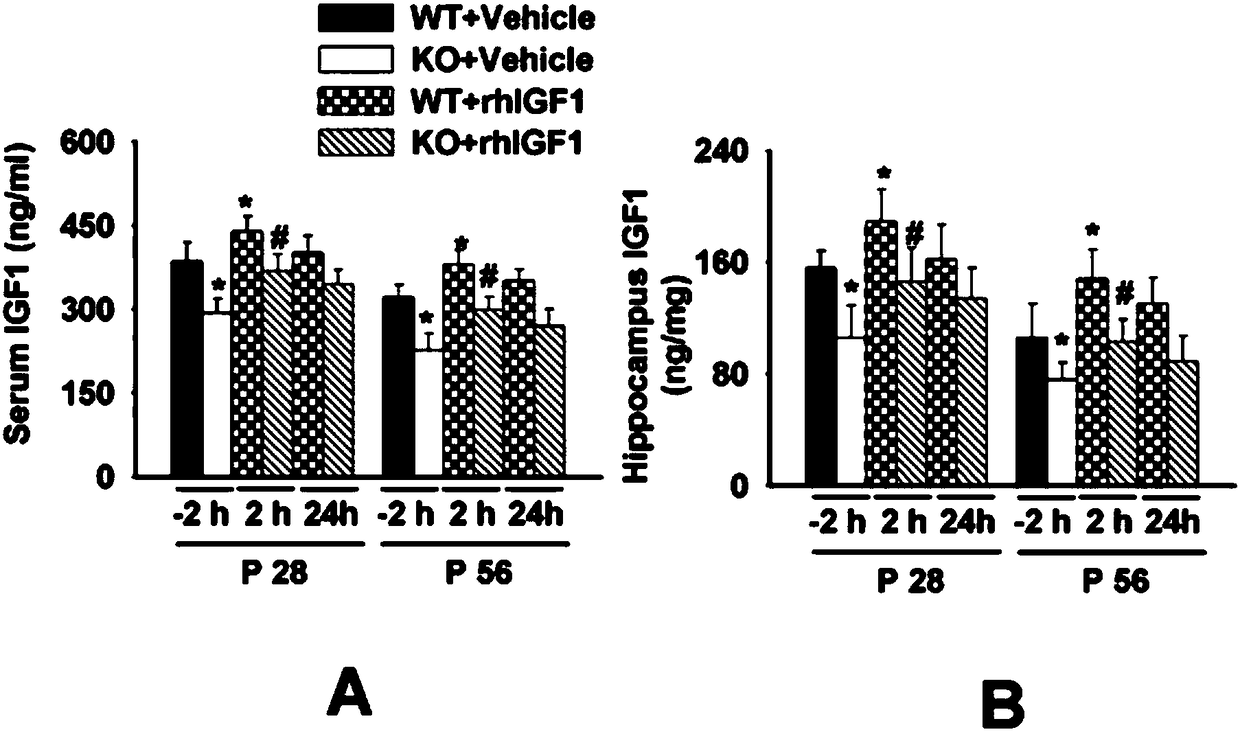
[0034] Example 1: The effect of long-term rhIGF1 treatment on the level of IGF1 in serum and hippocampus of FMR1KO mice
[0035] The present invention first detected the level of IGF1 in the hippocampus of the mouse serum and the key nucleus of learning and memory in the brain at 28 days (P28) and 56 days (P56) after birth, and whether long-term exogenous intraperitoneal injection of rhIGF1 (0.25 mg / kg) can Regulate the level of IGF1 in mouse serum and hippocampus. Take mice of the same age and different treatment groups at 28 and 56 days after birth. On the same day, before the injection of rhIGF1, 2h and 24h after the injection, blood was taken from the heart after anesthesia, left for 30min, centrifuged at 2000 rpm for 5min, and the serum was taken and frozen at -80℃. Check. After the mice were sacrificed, their brains were quickly decapitated, and the hippocampus was quickly separated and weighed separately. The lysate was added according to the tissue weight (mg): volume of...
Embodiment 2
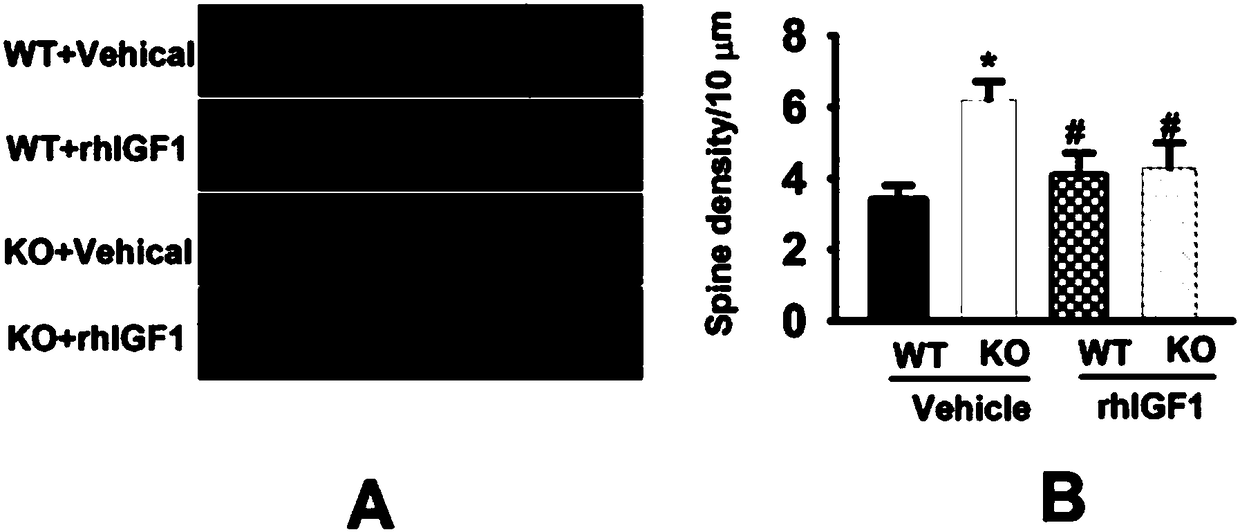
[0037] Example 2: Effect of long-term rhIGF1 treatment on the morphology of hippocampal neurons in FMR1KO mice
[0038] A large amount of evidence proves that the density of immature dendritic spine of hippocampal pyramidal neurons in FMR1KO mice is significantly increased. In the present invention, 5 mice of the same age are taken from each group, directly killed and washed with water. According to the instructions of the Golgi staining kit, 3 neurons are randomly selected, a total of 15 neurons, and dendrites of various levels within 100 μm from the cell body are selected. The number of total dendritic spines and thin dendritic spines with immature long necks and small heads on the 50μm dendrites were recorded using ImageJ software every 10μm.
[0039] See the result figure 2 ,by figure 2 According to the results in, at 56 days of birth (P56), the density of slender dendritic spine neurons in the hippocampal dentate gyrus of the KO+Vehicle group was significantly greater than t...
Embodiment 3
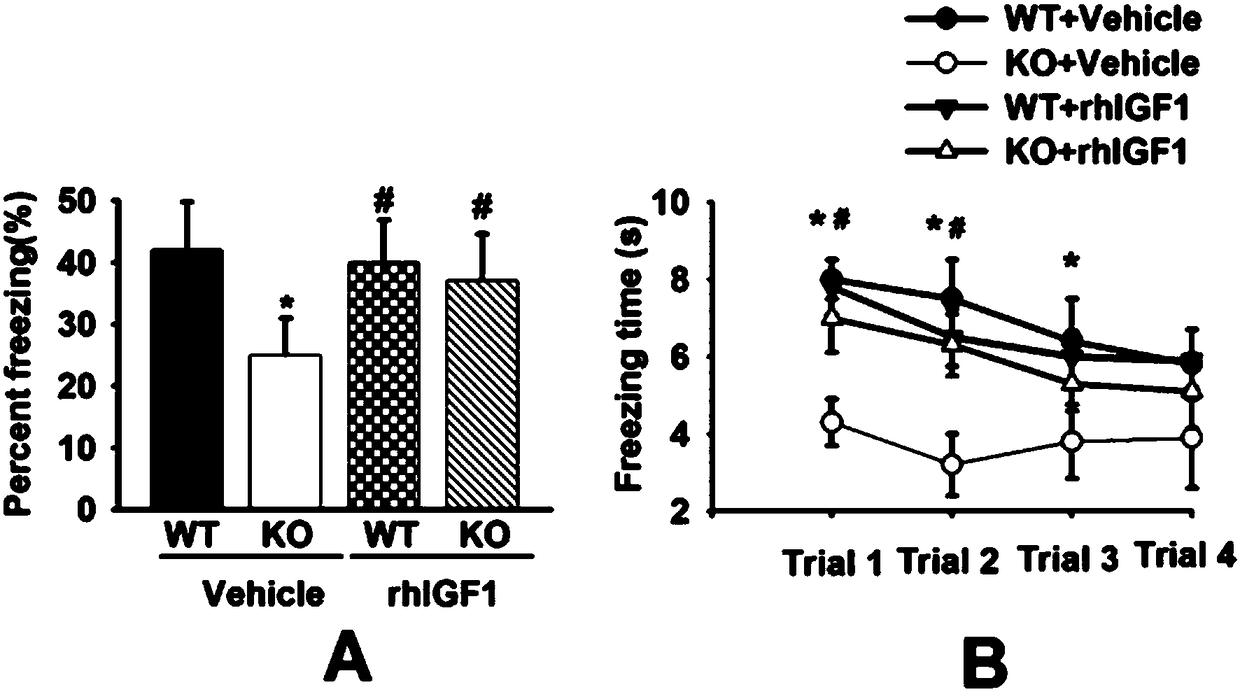
[0040] Example 3: Effect of long-term rhIGF1 treatment on anxiety-like behavior in FMR1KO mice
[0041] A large amount of evidence shows that FMR1KO mice exhibit obvious anxiety-like behaviors, as well as hyperactivity symptoms. The present invention uses the internationally recognized classic open field test (Open field test, OFT) elevated plus maze (Elevated plus-maze test, EPM) to detect anxiety-like behaviors in mice, and mainly uses mice to open the central area of the open field and the elevated plus maze. The nervousness of the conflict between the fear of the arm area and the desire to explore novelty is used to judge the anxiety of the mice. The more anxious the mice, the shorter the residence time in the central area of the open field and the open arm area of the Gaojia plus maze. At the same time, the stronger the mouse's exercise activity, the longer the movement distance in the two experimental devices, and the more times the arm moves.
[0042] The open field e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com