Lenalidomide crystal and preparation method thereof
A technology of lenalidomide and crystal, applied in the field of lenalidomide crystal and its preparation, can solve the problems of unmentioned solubility and improvement, and achieve easy operation and implementation, good solubility, and crystal stability. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 (preparation of new crystal form of lenalidomide)
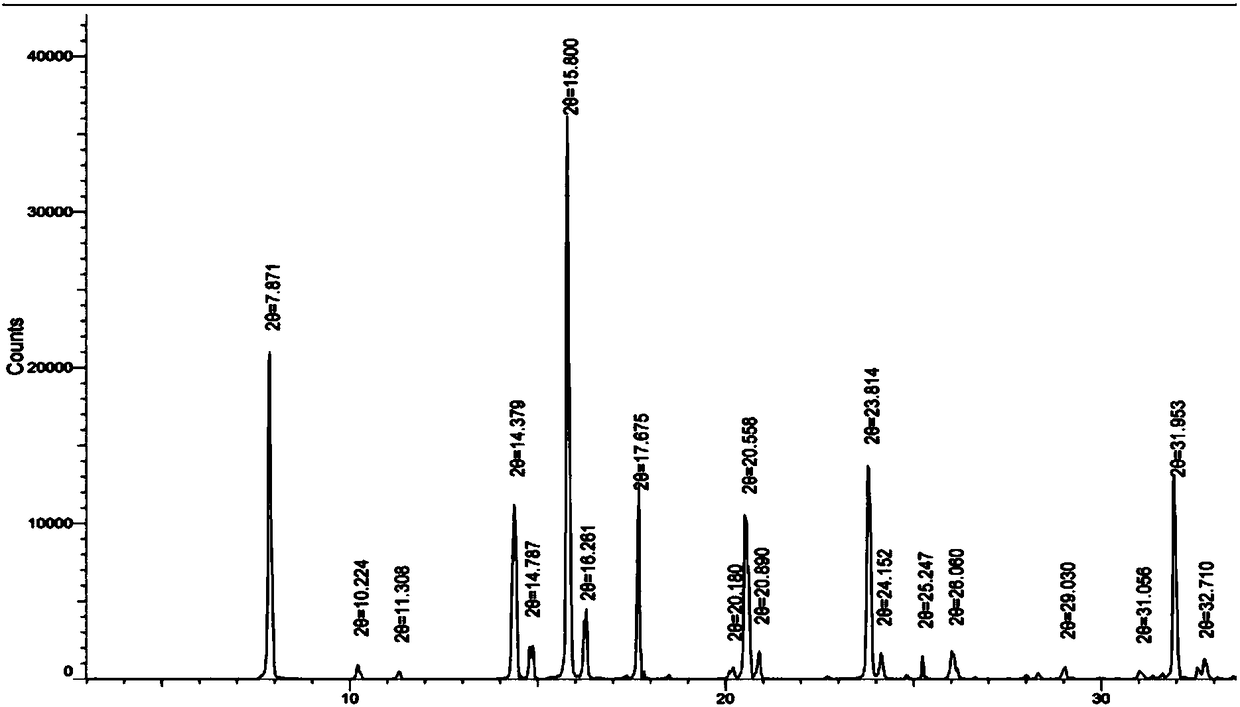
[0038] Add 1.1g of commercially available lenalidomide (Wuhan Yuancheng Gongchuang Technology Co., Ltd., batch number: 20160901) into a 250mL flask, add 120mL of anhydrous methanol, stir and heat to reflux, heat filter after dissolving, and place the filtrate in The crystallization was naturally cooled at room temperature of 30°C, the solid was collected by filtration, and dried under reduced pressure for two days at a temperature of 30°C, with a yield of 95.6%.
[0039] The obtained crystal sample is subjected to X-ray powder diffraction test, and the spectrum is shown in figure 1 (Using Bruker D8ADVANCE model X-ray powder diffractometer of German Bruker company to analyze the crystal phase of the sample, radiation source Cu Kα, scanning method: step scanning; primary Twin opitcs: 0.5° divergence; secondary Twin opitcs: fixed mm, 5.8 mm; scanning range: 3°~40°; scanning step length: 0.02°; dwell time of ...
Embodiment 2
[0043] Embodiment 2 (preparation of new crystal form of lenalidomide)
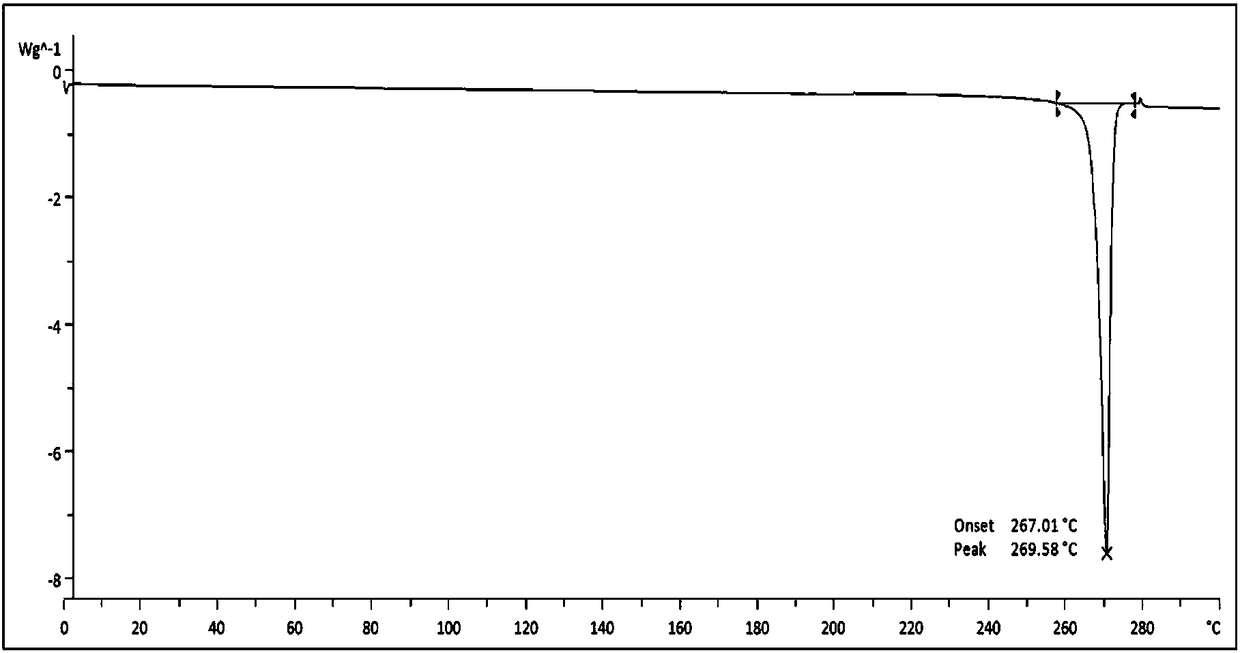
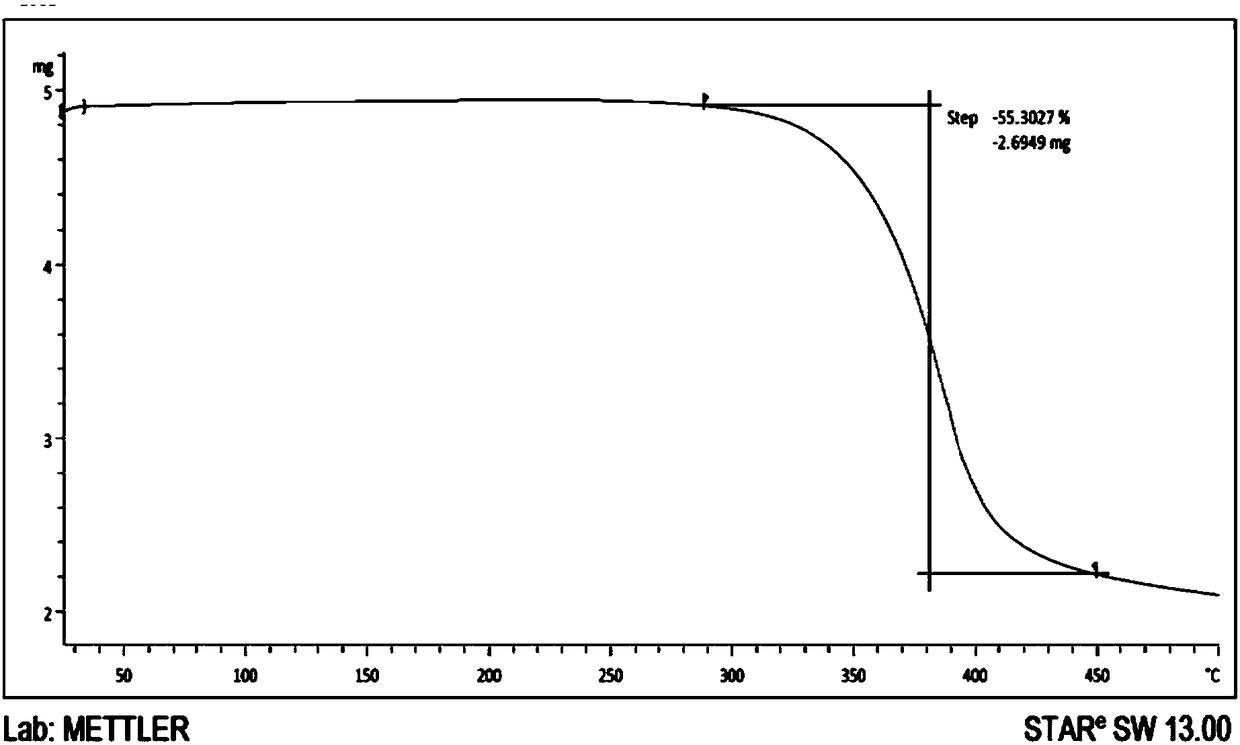
[0044] Add 1.1g of commercially available lenalidomide into a 250mL flask, add 120mL of anhydrous methanol, stir and heat to reflux, dissolve and filter hot, place the filtrate to cool at 40 degrees Celsius, filter and collect the precipitated solid, and depressurize After drying for two days, the yield was 89.1%. The X-ray powder diffraction, DSC, and TGA spectra of the crystal are basically consistent with those of Example 1.
Embodiment 3
[0045] Embodiment 3 (solubility test)
[0046] Test group: the new crystal form of lenalidomide prepared by the inventive method;
[0047] Control group: lenalidomide crystal form B prepared by referring to the method disclosed in the existing literature (CN1871003, US20050096351).
[0048] Instruments and reagents:
[0049] High performance liquid chromatography: SHIMADZU (SPD-20A)
[0050] Electronic balance: SARTORIUS (BP211D)
[0051] Oscillator: Incubator Shakers (HZ-9211KB)
[0052] purified water
[0053] Methanol (analytical pure)
[0054] Chromatographic conditions:
[0055] Chromatographic column: DikamaTechndogiesC18 (250*4.6mm, 4.6um)
[0056] Mobile phase: 0.05% formic acid aqueous solution: acetonitrile solution = 20:80
[0057] Detection wavelength: 254nm; flow rate: 1.0ml / min; column temperature: 35°C
[0058] Under the above-mentioned chromatographic conditions, the retention times of lenalidomide series compounds are shown in Table 2:
[0059] Table...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com