Apostichopus japonicus F type lectin AjFL-1 as well as preparation method and application
A technology of lectin and japonicus imitation, which is applied in the field of molecular biology and can solve the problems of no preparation method of japonicus japonicus f-type lectin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
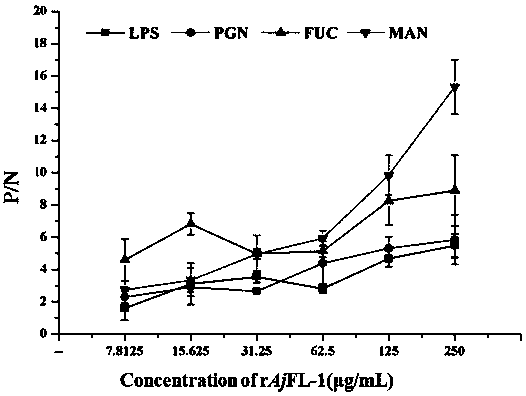
[0038] Apostichopus japonicus F-type lectin of the present invention Aj The binding activity detection of FL-1 and different PAMPs (LPS, PGN, Mannan and Fucose), the specific steps are as follows:
[0039] (1) Coating solution (50 mM Na 2 CO 3 -NaHCO 3 buffer, pH 9.8) to dilute the different PAMPs to 100 μgmL -1 , add 100 μL per well into a 96-well microtiter plate, and coat overnight at 4 °C;
[0040] (2) Wash 3 times with TBST, 5 min each time;
[0041] (3) Add 200 μL of 3% BSA solution (dissolved in TBST) to each well, and block in a 37 °C incubator for 1 h;
[0042](4) Wash 3 times with TBST, 5 min each time;
[0043] (5) Apostichopus japonicus F-type lectin of the present invention Aj After FL-1 was serially diluted to two-fold concentration, 100 μL per well (three parallels were set for each gradient, TBST was used as a blank control, and the tagged protein Trx was used as a negative control), and incubated at 18 °C for 3 h;
[0044] (6) Wash 3 times with TBST, 5...
experiment example 2
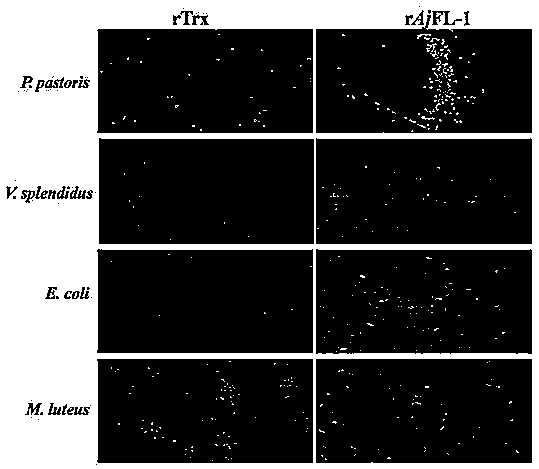
[0053] Apostichopus japonicus F-type lectin of the present invention Aj FL-1 and different bacteria ( P. pastoris , V. splendidus, E. coli, M. luteus ) agglutination activity assay. Escherichia coli ( Escherichia coli Top10, purchased from Beijing Tiangen Biochemical Technology Co., Ltd.), Vibrio splendidus ( Vibrio splendidus , purchased from Beijing Microorganism Culture Collection Center), Pichia pastoris ( Pichia pastoris GS115, purchased from Invitrogen), Micrococcus luteus ( Micrococcus luteus , purchased from Beijing Microorganism Culture Collection Center). Specific steps are as follows:
[0054] (1) FITC staining: the cells were collected by centrifugation at 5000 rpm for 5 min. After the medium was discarded, TBS buffer was added, and the cells were washed three times. Add FITC (final concentration 0.1mg ml -1 ), stained in the dark for 30 min. Cells were collected by centrifugation at 5000 rpm for 5 min. After discarding the supernatant, add TBS ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com