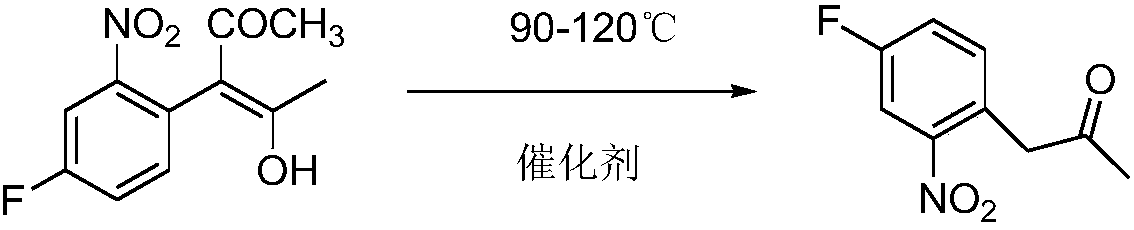
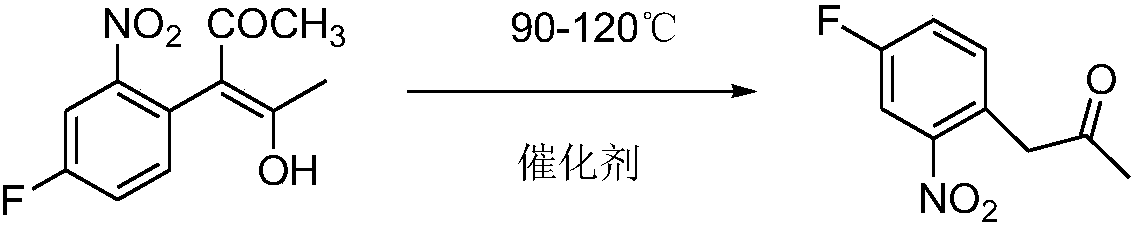
Method used for preparing 3-(4-fluoro-2-nitrophenyl) propanone
A technology of nitrophenyl and acetone, applied in the field of preparing 3-acetone, can solve the problems of large amount of acid-containing waste water, large amount of sulfuric acid used, etc., and achieves the advantages of improving product content and yield, easy operation, and reducing waste liquid amount. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the reaction flask of 500ml, add 240g acetic acid, 41.2g 3-(4-fluoro-2-nitrophenyl)-4-hydroxyl-3-penten-2-ketone, 10g resin catalyst D001, stir and gradually heat up to 110°C, react for 3 hours, liquid chromatography tracking 3-(4-fluoro-2-nitrophenyl)-4-hydroxy-3-penten-2-one content <0.2%, the reaction is over. The reaction solution was filtered while it was hot, and the catalyst was washed with 20 g of acetic acid. About 2 / 3 of the solvent was removed from the filtrate, and the temperature was lowered to 25-30 ° C. A large amount of solids were precipitated. After filtering, the filter cake was dried to obtain 32.2 g of 3-(4-fluoro-2-nitrate phenyl) acetone, content 95%, yield 90%, the filtrate was precipitated, solvent and catalyst were directly applied to the next batch, residue 8g. This method 100g product produces 25g organic residue, 1g spent catalyst (catalyst is calculated according to using 30 discardings), and this step reaction does not produce waste gas...
Embodiment 2
[0024] In the reaction flask of 1000ml, add 400g acetic acid, 82.4g 3-(4-fluoro-2-nitrophenyl)-4-hydroxyl-3-penten-2-one, 20g resin catalyst D002, stir and gradually warm up to 112°C, react for 3 hours, liquid chromatography tracking 3-(4-fluoro-2-nitrophenyl)-4-hydroxy-3-penten-2-one content <0.2%, the reaction is over. The reaction solution was filtered while it was hot, and the catalyst was washed with 40 g of acetic acid. About half of the solvent was removed from the filtrate, and the temperature was lowered to 25-30 ° C. A large amount of solids were precipitated. After filtering, the filter cake was dried to obtain 66.9 g of 3-(4-fluoro-2-nitrobenzene Base) acetone, content 97%, yield 95.5%, filtrate precipitation, solvent and catalyst are directly applied to the next batch, residue 12g. 100g of this method produces 18g of organic residues and 1g of spent catalyst (catalyst is discarded according to 30 times of use), and this step reaction does not produce waste gas and...
Embodiment 3
[0026] Add 500g acetic acid, 82.4g3-(4-fluoro-2-nitrophenyl)-4-hydroxyl-3-penten-2-one, 40g resin catalyst D61 in a 1000ml reaction flask, stir and gradually heat up to 90 °C, reacted for 6 hours, liquid chromatography traced the content of 3-(4-fluoro-2-nitrophenyl)-4-hydroxy-3-penten-2-one <0.2%, the reaction ended. The reaction solution was filtered while it was hot, and the catalyst was washed with 40 g of acetic acid. About 2 / 3 of the solvent was removed from the filtrate, and the temperature was lowered to 25-30 ° C. A large amount of solids were precipitated. After filtering, the filter cake was dried to obtain 65.8 g of 3-(4-fluoro-2-nitrate phenyl) acetone, content 95%, yield 92%, the filtrate precipitation, solvent and catalyst directly applied to the next batch, residue 15g. 100g of this method produces 23g of organic residues and 2g of spent catalyst (the catalyst is discarded according to 30 times of use), and this step reaction does not produce waste gas and wast...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com