Method for preparing charge-driven self-assembled chitosan-based drug-loaded nanoparticles
A drug-loaded nano and chitosan technology, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, and medical preparations containing active ingredients. Toxicity and other issues, achieve good biocompatibility and biomedical prospects, reduce the risk of solvent residues, and controllable size and potential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
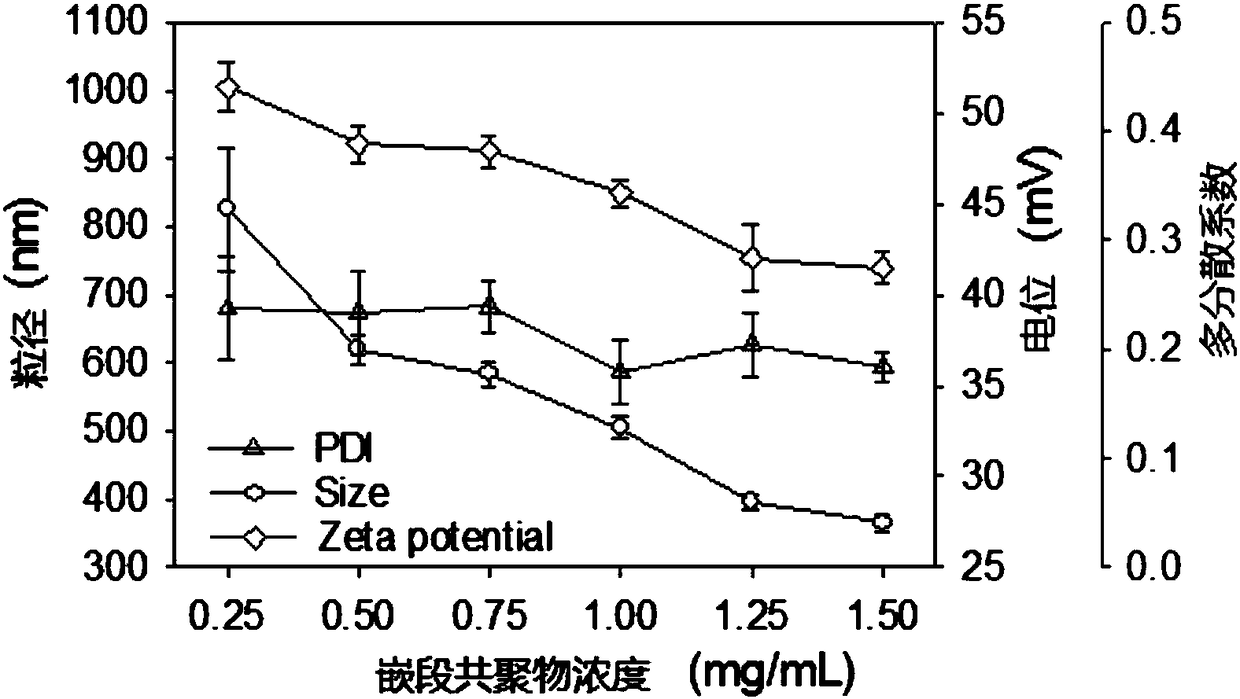
[0043] Embodiments of the present invention include the following steps:
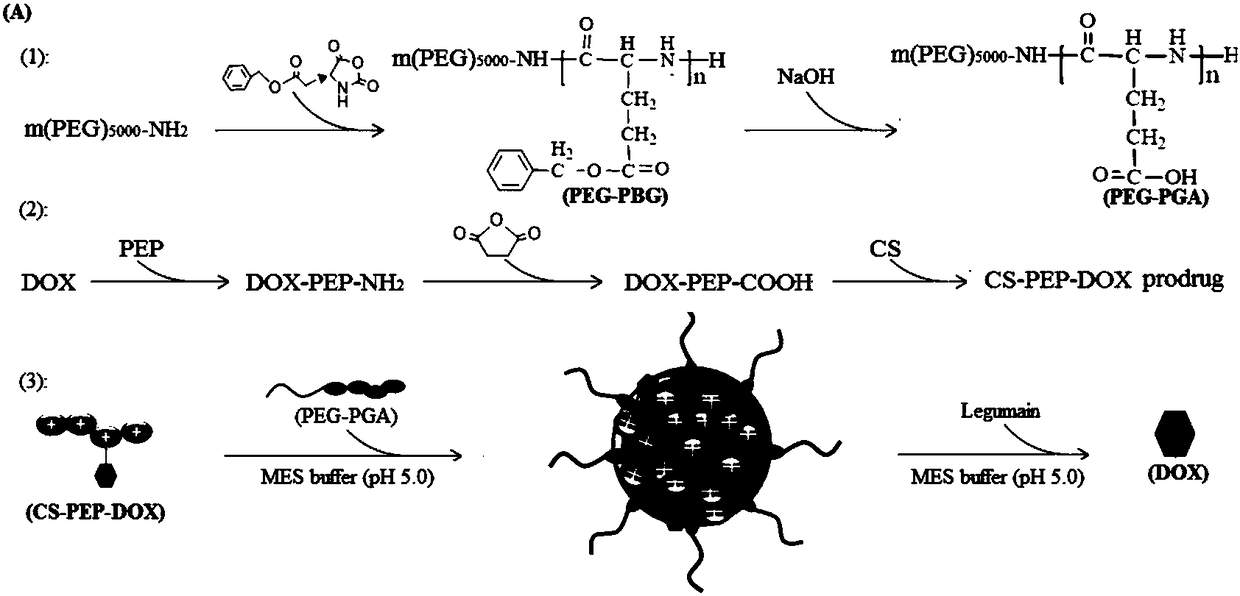
[0044] (1) Preparation of PEG-PGA amphoteric dissociation copolymer:
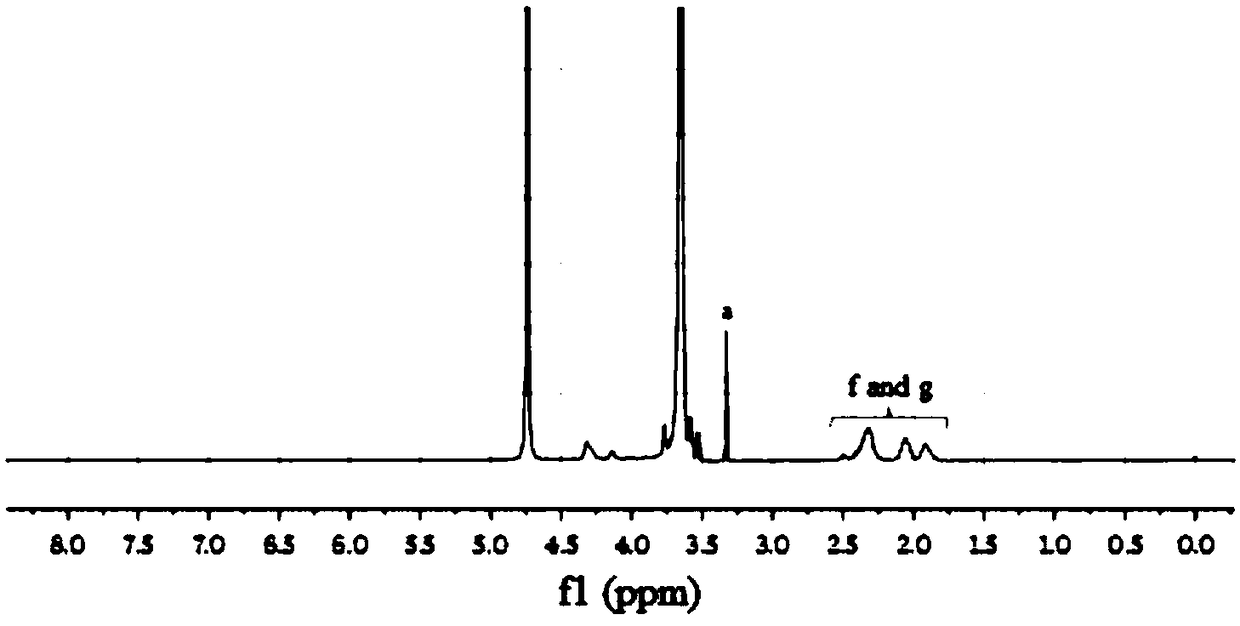
[0045]Benzyl glutamate-N-carbonyl intracyclic anhydride induced by amino PEG to form a block copolymer of PEG and polybenzyl glutamate, which was hydrolyzed under basic conditions to remove polybenzyl glutamate The terminal benzyl group forms a block copolymer of PEG-PGA. The synthesis example is as follows: 1.2 g of benzyl glutamate-N-carbonyl ring anhydride was dissolved in 20 mL of dry nitrogen nitrogen dimethylformamide, mixed with amino PEG and reacted at 38 degrees for 48 hours under nitrogen protection. The block copolymer of PEG and polybenzyl glutamate was obtained by dialysis, and the benzyl group was removed under the action of NaOH to form a PEG-PGA block copolymer. see figure 2 As stated, it can be confirmed that the PEG-PGA block copolymer has been successfully synthesized, and its molecular structural formula is: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com