Method for synthesizing lornoxicam intermediate by one-pot method
A technology for lornoxicam and intermediates, which is applied in the field of one-pot synthesis of lornoxicam intermediates, can solve problems such as complicated operation process, and achieve the effects of improving product yield, cheap reagents, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
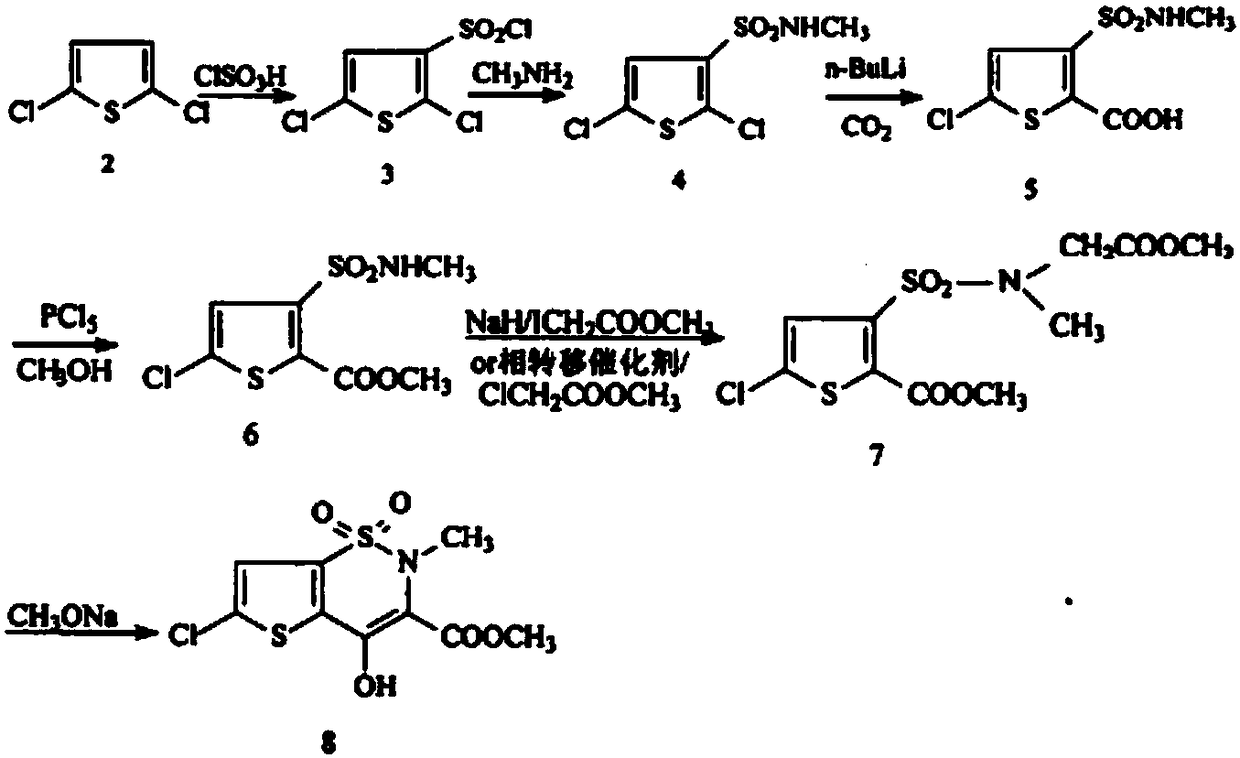
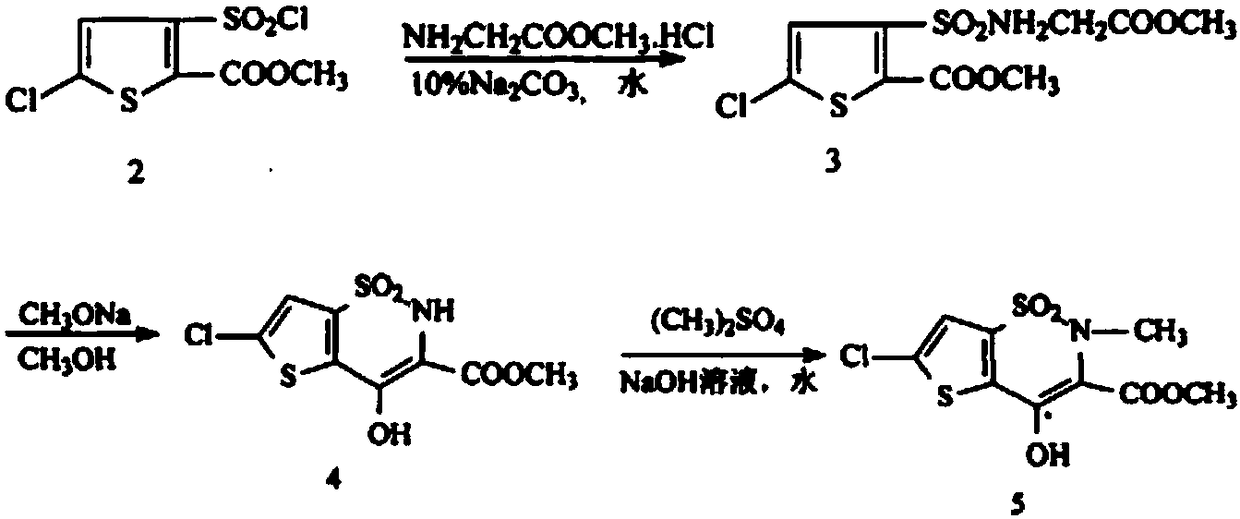
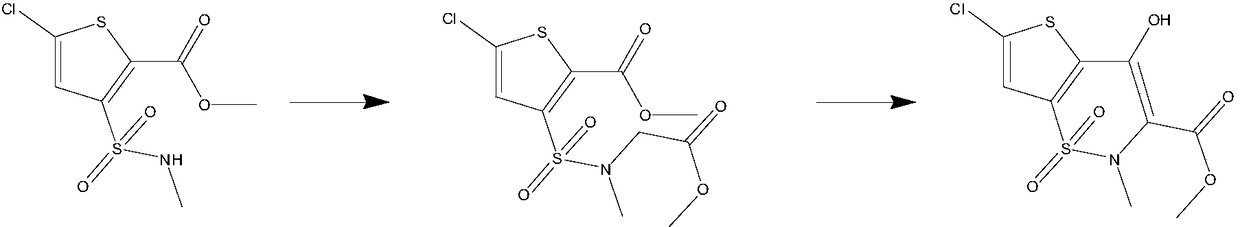
[0033] N 2 Add 100ml of methanol, 10g of methyl 5-chloro-3-methylsulfonamidethiophene-2-carboxylate, and 6.82g of methyl bromoacetate into a 250ml four-necked flask under protection, stir until completely dissolved, and add 28wt.% methanol dropwise Sodium methanol solution 8g, slightly exothermic, the color of the solution turns yellow, stir at room temperature 25°C, and react for 24 hours; then add 8g of 28wt.% sodium methoxide methanol solution dropwise, the solution color changes from yellow to orange red, stir at room temperature 25°C , reacted for 12 hours; then ice-thawed, adjusted the pH of the solution to 4 with concentrated hydrochloric acid, extracted three times with dichloromethane, combined the organic phases, washed once with water, dried over anhydrous magnesium sulfate, filtered, concentrated to obtain a light yellow solid, and recrystallized from methanol , filtered, and dried under vacuum to obtain 6-chloro-4-hydroxy-2-methyl-2H-thieno[2,3-e]-1,2-thiazine-3-c...
Embodiment 2
[0035] N 2 Add 100ml of methanol, 10g of methyl 5-chloro-3-methylsulfonamide thiophene-2-carboxylate, and 12.4g of methyl bromoacetate into a 250ml four-neck flask under protection, stir until completely dissolved, and add 28wt.% methanol dropwise 14g of sodium methanol solution, slightly exothermic, the color of the solution turned yellow, stirred at 45°C, and reacted for 12 hours; then 14g of 28wt.% sodium methylate methanol solution was added dropwise, the color of the solution changed from yellow to orange red, stirred at room temperature at 25°C, Reacted for 12 hours; then ice-thawed, adjusted the pH of the solution to 4 with concentrated phosphoric acid, extracted three times with dichloromethane, combined the organic phases, washed once with water, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a light yellow solid, which was then recrystallized from methanol. Filter and dry in vacuum to get 6-chloro-4-hydroxy-2-methyl-2H-thieno[2,3-e]-1,2-...
Embodiment 3
[0037] N 2 Add 200ml of methanol, 20g of methyl 5-chloro-3-methylsulfonamide thiophene-2-carboxylate, and 13.64g of methyl bromoacetate into a 500ml four-neck flask under protection, stir until completely dissolved, and add 28wt.% methanol dropwise 16g of sodium methanol solution, slightly exothermic, the color of the solution turns yellow, stir and react at 50°C for 12 hours; then add 16g of 28wt.% sodium methoxide methanol solution dropwise, the color of the solution changes from yellow to orange red, stir and react at 50°C 6 hours; then ice-thawed, adjusted the pH of the solution to 4 with dilute sulfuric acid, extracted three times with ethyl acetate, combined the organic phases, washed once with water, dried over anhydrous magnesium sulfate, filtered, and concentrated to obtain a light yellow solid, then recrystallized with methanol, filtered , and dried in vacuum to obtain 6-chloro-4-hydroxy-2-methyl-2H-thieno[2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester-1,1-di Ox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com