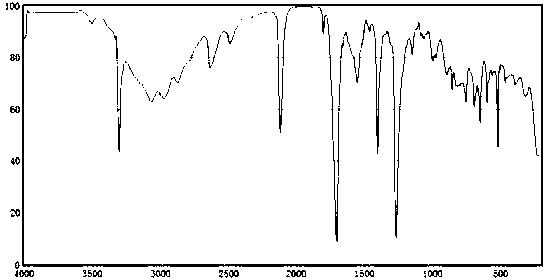
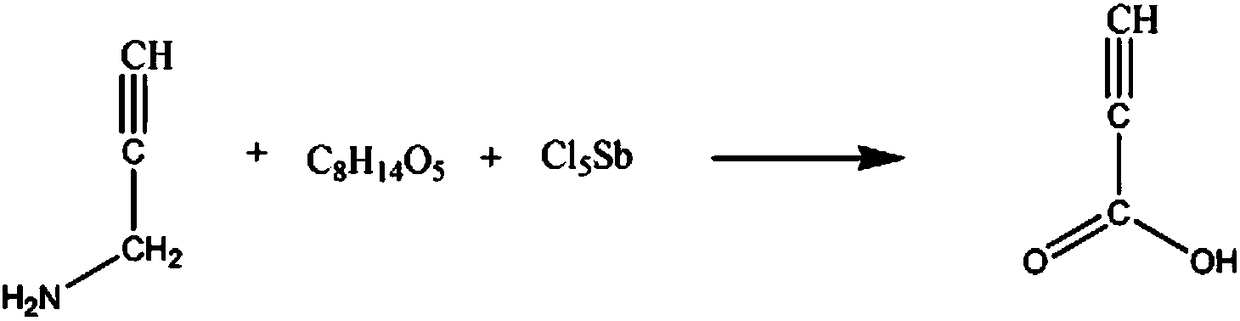Synthetic method for flavonoid glycoside drug intermediate propiolic acid
A synthesis method and technology of propiolic acid, applied in chemical instruments and methods, preparation of organic compounds, preparation of carboxylate, etc., can solve the problems of increased risk factor, large environmental pollution, high processing cost, etc., and achieve an increase in reaction yield , avoid pollution, improve the effect of reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The synthetic method of flavone glycoside drug intermediate propiolic acid comprises the steps:
[0019] A: Add 2mol 3-aminopropyne and 1.5L of sodium nitrate solution with a mass fraction of 10% in the reaction vessel, control the stirring speed at 210rpm, control the solution temperature to 25°C, react for 60min, and add 800ml of 20% sodium nitrate solution with a mass fraction of Base tert-butyl ether solution, raise the temperature of the solution to 40°C, and react for 1h;
[0020] B: Add 2 mol of antimony pentachloride powder, 4 mol mass fraction of 40% diglycol diacetate solution, increase the solution temperature to 50 ° C, react for 30 min, extract 3 times with ethyl tert-butyl ether solution, static Set aside for 2h, the solution was separated into layers, and the oil layer was separated, washed 5 times in a potassium chloride solution with a mass fraction of 15%, and washed 6 times in a 3-methyl-2-pentanone solution with a mass fraction of 50%. Washed 8 time...
Embodiment 2
[0022] The synthetic method of flavone glycoside drug intermediate propiolic acid comprises the steps:
[0023] A: Add 2mol 3-aminopropyne and 1.5L of sodium nitrate solution with a mass fraction of 13% in the reaction vessel, control the stirring speed at 220rpm, control the solution temperature to 27°C, react for 70min, and add 800ml of 25% sodium nitrate solution with a mass fraction of Base tert-butyl ether solution, raise the temperature of the solution to 43°C, and react for 1.5h;
[0024] B: Add 2.5 mol of antimony pentachloride powder, 4.5 mol of diglycol diacetate solution with a mass fraction of 43%, raise the temperature of the solution to 52°C, react for 40 minutes, and extract 4 times with ethyl tert-butyl ether solution , standing for 2.5h, the solution was separated, and the oil layer was separated, washed 6 times in a potassium chloride solution with a mass fraction of 18%, and washed 7 times in a 3-methyl-2-pentanone solution with a mass fraction of 54%. , wa...
Embodiment 3
[0026] The synthetic method of flavone glycoside drug intermediate propiolic acid comprises the steps:
[0027] A: Add 2mol 3-aminopropyne and 1.5L of sodium nitrate solution with a mass fraction of 16% in the reaction vessel, control the stirring speed at 230rpm, control the solution temperature to 30°C, react for 80min, and add 800ml of 27% sodium nitrate solution with a mass fraction of Base tert-butyl ether solution, raise the temperature of the solution to 45°C, and react for 2h;
[0028] B: Add 3mol antimony pentachloride powder, 5mol mass fraction of 46% diglycol diacetate solution, increase the solution temperature to 56°C, react for 50min, extract 5 times with ethyl tert-butyl ether solution, static Set aside for 2-3h, the solution was separated, and the oil layer was separated, washed 7 times in a potassium chloride solution with a mass fraction of 22%, and washed 8 times in a 3-methyl-2-pentanone solution with a mass fraction of 55%. Washing 10 times in dipropylene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com