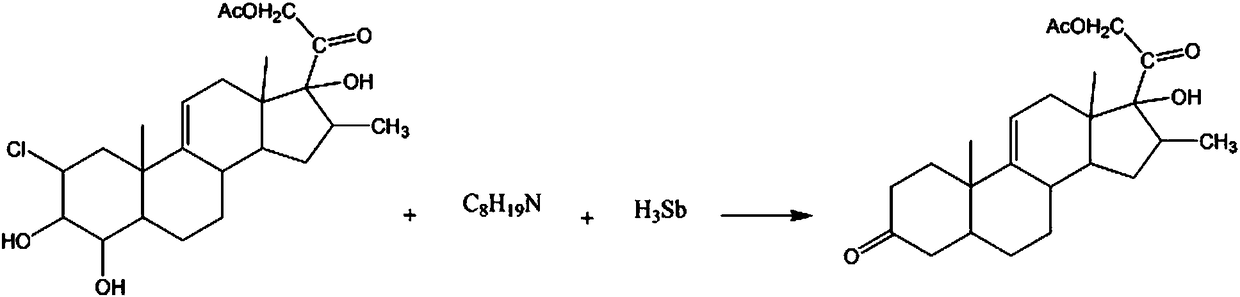
Synthetic method for hormone drug intermediate 9(11)-pregnene-16beta-methyl-17alpha,21-diol-3,20-dione-21-acetate
A synthetic method, the technology of pregnene, which is applied in the field of preparation of pharmaceutical intermediates, can solve the problems of unsuitability for large-scale industrial production, complex synthesis process, and increased risk, so as to shorten the reaction time, increase the reaction yield, The effect of avoiding pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The synthetic method of hormone drug intermediate 9(11)-pregnene-16β-methyl-17α, 21-diol-3,20-diketone-21-acetate comprises the following steps:
[0018] A: Add 3mol 2-chloro-4-hydroxy-9(11)-pregnene-16β-methyl-3β,17α,21-triol-20-keto-21-acetate in the reaction vessel, 1.6L A potassium chloride solution with a mass fraction of 15%, a 2-picoline solution with a mass fraction of 30%, lower the temperature of the solution to 10° C., control the stirring speed at 210 rpm, and continue the reaction for 30 min;
[0019] B: Add 6 mol of diisobutylamine solution with a mass fraction of 40%, raise the solution temperature to 30°C, react for 50min, add 2mol of antimony hydrogen powder, raise the temperature to 40°C, react for 30min, let stand for 1h, filter, Obtain crystal, wash with the sodium nitrate solution that mass fraction is 10%, the benzonitrile solution that mass fraction is 40% washs, the p-bromochlorobenzene solution that mass fraction is 50% washs, the diethylene gly...
Embodiment 2
[0021] The synthetic method of hormone drug intermediate 9(11)-pregnene-16β-methyl-17α, 21-diol-3,20-diketone-21-acetate comprises the following steps:
[0022] A: Add 3mol 2-chloro-4-hydroxy-9(11)-pregnene-16β-methyl-3β,17α,21-triol-20-keto-21-acetate in the reaction vessel, 1.6L A potassium chloride solution with a mass fraction of 18%, a 2-picoline solution with a mass fraction of 33%, lower the temperature of the solution to 14° C., control the stirring speed at 220 rpm, and continue the reaction for 40 min;
[0023] B: Add 6.5mol of diisobutylamine solution with a mass fraction of 44%, raise the temperature of the solution to 33°C, react for 65min, add 2.5mol of antimony hydrogen powder, raise the temperature to 42°C, react for 35min, and let stand for 1.5h , filtered to obtain crystals, washed with 14% sodium nitrate solution with a mass fraction, washed with a 45% benzonitrile solution, washed with a 53% p-bromochlorobenzene solution, and washed with a 75% di Recrystal...
Embodiment 3
[0025] The synthetic method of hormone drug intermediate 9(11)-pregnene-16β-methyl-17α, 21-diol-3,20-diketone-21-acetate comprises the following steps:
[0026] A: Add 3mol 2-chloro-4-hydroxy-9(11)-pregnene-16β-methyl-3β,17α,21-triol-20-keto-21-acetate in the reaction vessel, 1.6L A potassium chloride solution with a mass fraction of 22%, a 2-picoline solution with a mass fraction of 36%, lower the temperature of the solution to 15° C., control the stirring speed at 230 rpm, and continue the reaction for 50 min;
[0027] B: Add 7 mol of diisobutylamine solution with a mass fraction of 45%, raise the solution temperature to 36°C, react for 80min, add 3mol of antimony hydrogen powder, raise the temperature to 45°C, react for 40min, let stand for 2h, filter, Obtain crystal, be that the sodium nitrate solution washing of 16% with mass fraction, the benzonitrile solution washing that mass fraction is 47%, the p-bromochlorobenzene solution washing that mass fraction is 56%, the diet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com