Preparation method and use of micromolecule water reducer with delayed coagulation and slump retaining properties
A small-molecule water-reducing agent technology, applied in the field of preparation of small-molecule water-reducing agents, can solve problems such as high cost and poor economy, and achieve the effects of low production energy consumption, low raw material cost, and excellent concrete workability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
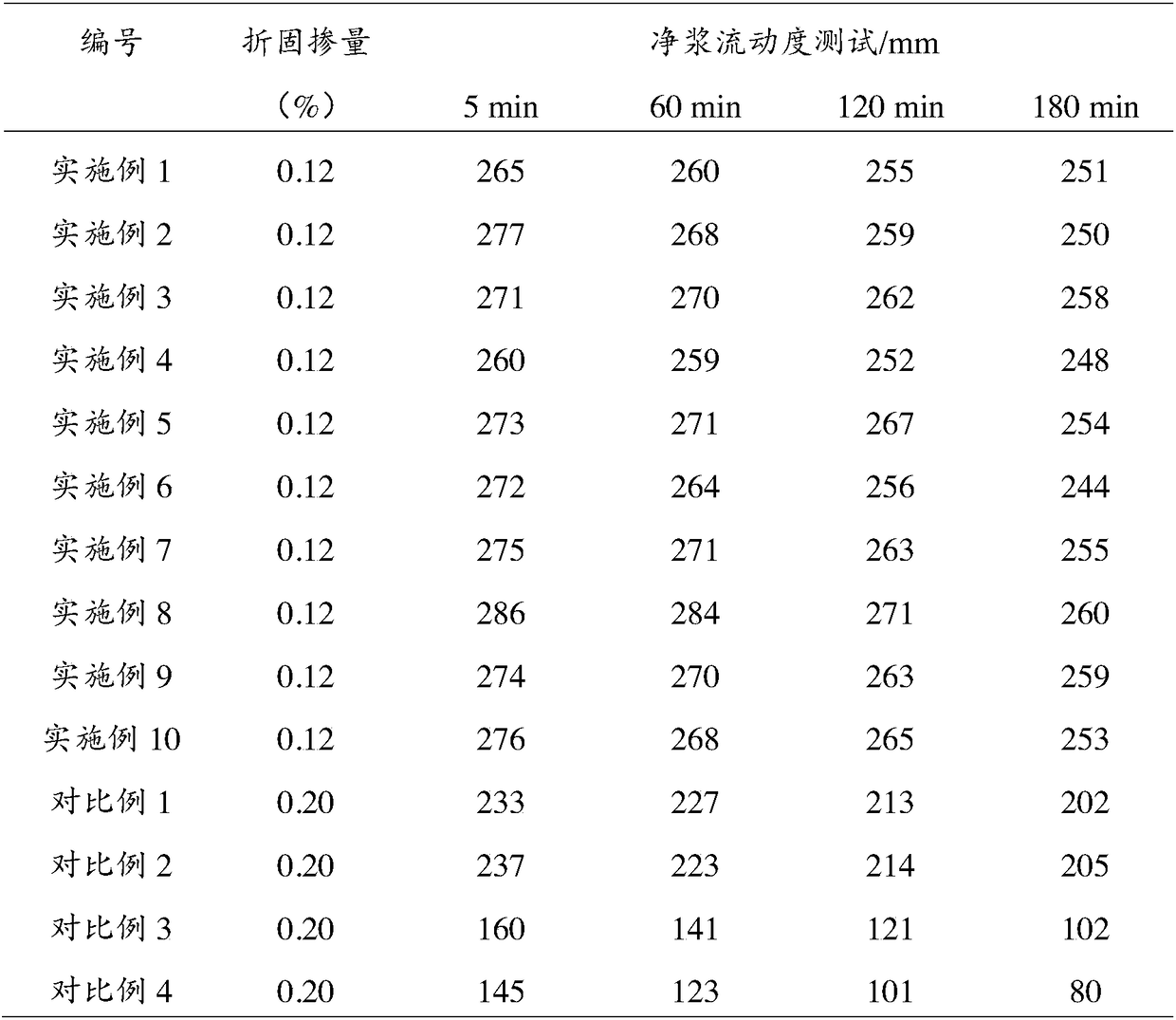
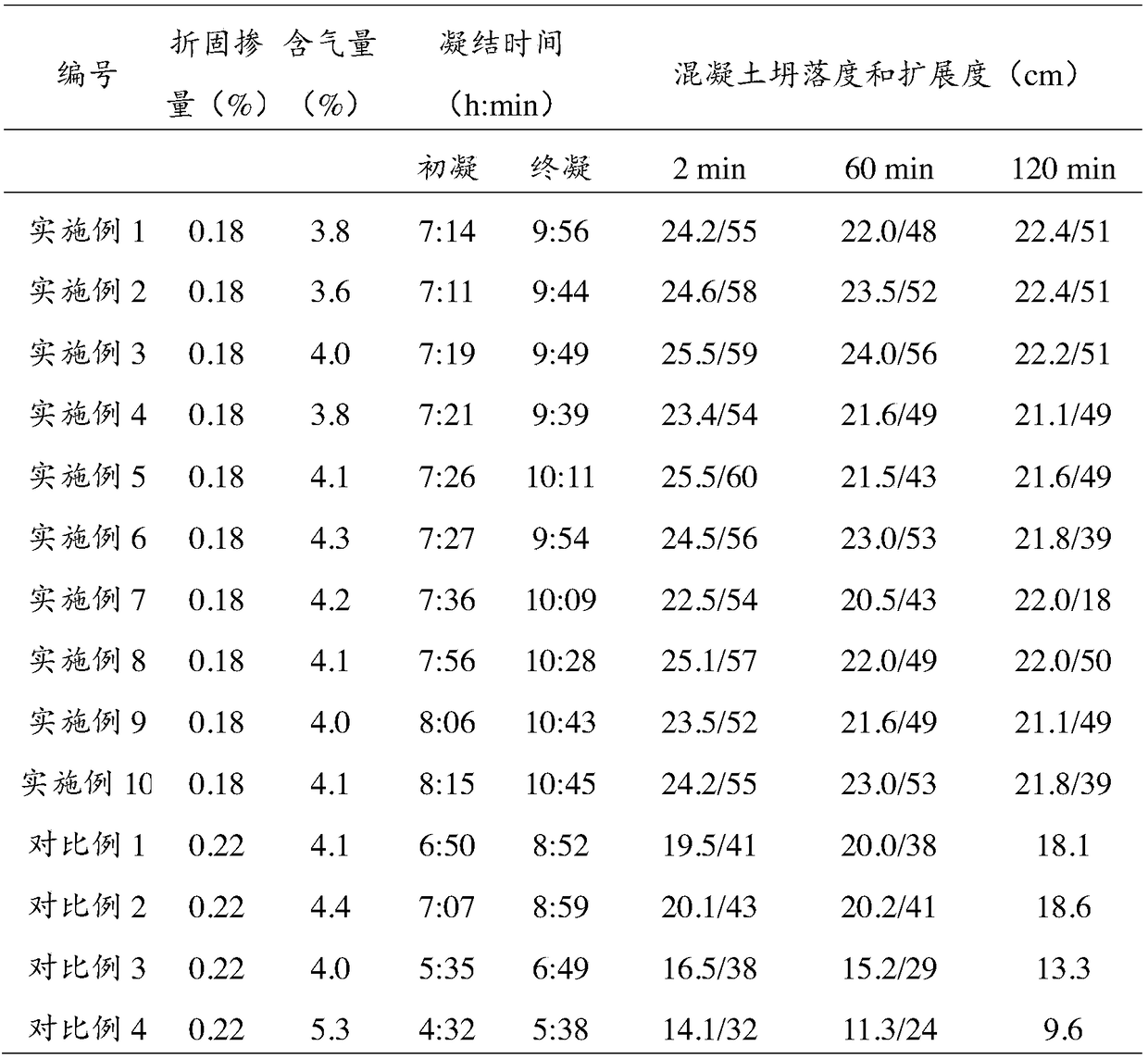
Image
Examples
Embodiment 1
[0065] (1) Amination reaction
[0066]Put 59.11g (1.0mol) of propylamine, 87.82g (1.02mol) of methyl acrylate and 0.68g of NKC-9 in a 250ml reaction bottle, raise the temperature to 40°C, stir and react for 1h, after the reaction is completed, remove the excess by vacuuming and vacuuming The methyl acrylate is removed by filtration to obtain an organic amine intermediate with a carboxylate group structure. By controlling the amount of organic amine and unsaturated carboxylic acid ester to be close to equimolar, a secondary amine intermediate formed by only one amino hydrogen participating in the addition reaction is obtained. The nucleophilic activity of the amine is greatly reduced and cannot continue to react with a slight excess of unsaturated carboxylate, so only one amino hydrogen may participate in the addition reaction.
[0067] (2) phosphorylation reaction
[0068] Put 145.20g (1.0mol) of the above-mentioned organic amine intermediate and 82.82g (1.01mol) of phosphor...
Embodiment 2
[0072] (1) Amination reaction
[0073] Put 269.51g (1.0mol) of octadecylamine, 87.82g (1.02mol) of methyl acrylate and 2.89g of NKC-9 in a 500ml reaction bottle, raise the temperature to 80°C, stir and react for 4h, after the reaction is completed, vacuumize and evaporate Excess methyl acrylate is removed, and insoluble matter is removed by filtration to obtain an organic amine intermediate with a carboxylate group structure. By controlling the amine and the unsaturated carboxylic acid ester to be close to equimolar amounts, a secondary amine intermediate formed by only one amino hydrogen participating in the addition reaction is obtained. The nucleophilic activity is greatly reduced and cannot continue to react with a slight excess of unsaturated carboxylic acid esters, so only one amino hydrogen may participate in the addition reaction.
[0074] (2) phosphorylation reaction
[0075] Put 355.60g (1.0mol) of the above-mentioned organic amine intermediate and 82.82g (1.01mol)...
Embodiment 3
[0079] (1) Amination reaction
[0080] Put 93.13g (1.0mol) of aniline, 103.12g (1.03mol) of 2-methyl methacrylate and 0.95g NKC-9 in a 500ml reaction bottle, raise the temperature to 80°C, stir and react for 6h, and vacuumize under reduced pressure after the reaction is completed The excess 2-methyl methacrylate was evaporated, and the insoluble matter was removed by filtration to obtain an organic amine intermediate with a carboxylate group structure. By controlling the amine and the unsaturated carboxylic acid ester to be close to equimolar amounts, a secondary amine intermediate formed by only one amino hydrogen participating in the addition reaction is obtained. The nucleophilic activity is greatly reduced and cannot continue to react with a slight excess of unsaturated carboxylic acid esters, so only one amino hydrogen may participate in the addition reaction.
[0081] (2) phosphorylation reaction
[0082] Put 193.25g (1.0mol) of the above-mentioned organic amine interm...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com