Pseudomonas aeruginosa and pseudomonas aeruginosa-containing marine mammal vaccine
A technology of Pseudomonas aeruginosa and mammals, applied in the field of vaccines, to achieve the effect of effective homologous attack protection and strong immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1, acquisition and preservation of bacterial strains
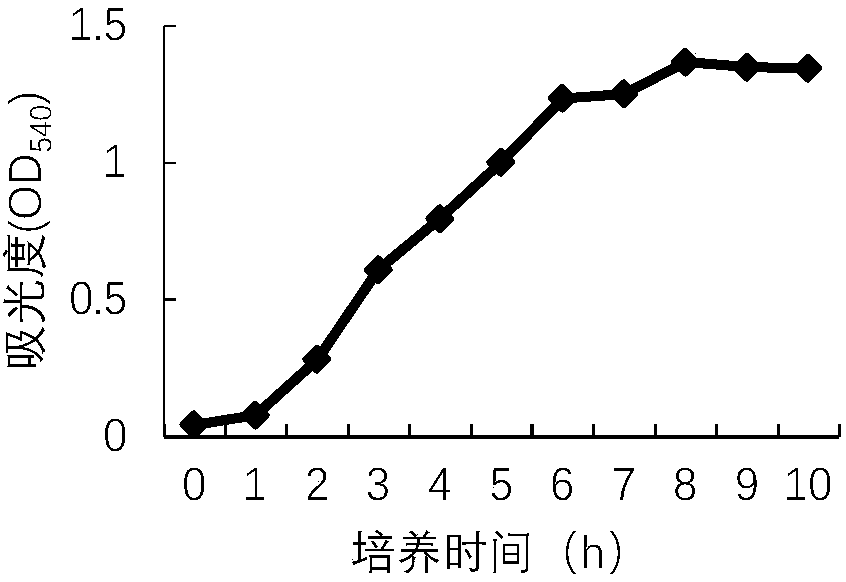
[0033] 1. From a large aquarium, the stomata of marine mammals were aseptically collected in sterile plastic vials, and streaked and inoculated on hexadecanetrimethylammonium bromide agar medium (NAC). Take round, smooth, moist, and flat colonies for Gram staining and microscopic examination, select Gram-negative small bacilli for streaking and pure culture, and conduct identification according to the biochemical identification test requirements for Pseudomonas aeruginosa, and further purify and cultivate The strains were grown in pyocyanin medium, oxidase test, mannitol, nitrate reduction test, gelatin liquefaction test, 42°C growth test, etc. The results are shown in Table 1. The strain was named Pseudomonas aeruginosa DCP1 strain.
[0034] Table 1 Biochemical test results of isolated strains
[0035] Biochemical test items
result
Biochemical test items
result
Pyocyanin me...
Embodiment 2
[0058] Embodiment 2, the preparation of vaccine
[0059] Thaw the frozen bacterial solution obtained in step 2 of Example 1 at room temperature, and perform the following steps on the P2 generation strain:
[0060] 1. Antigen preparation
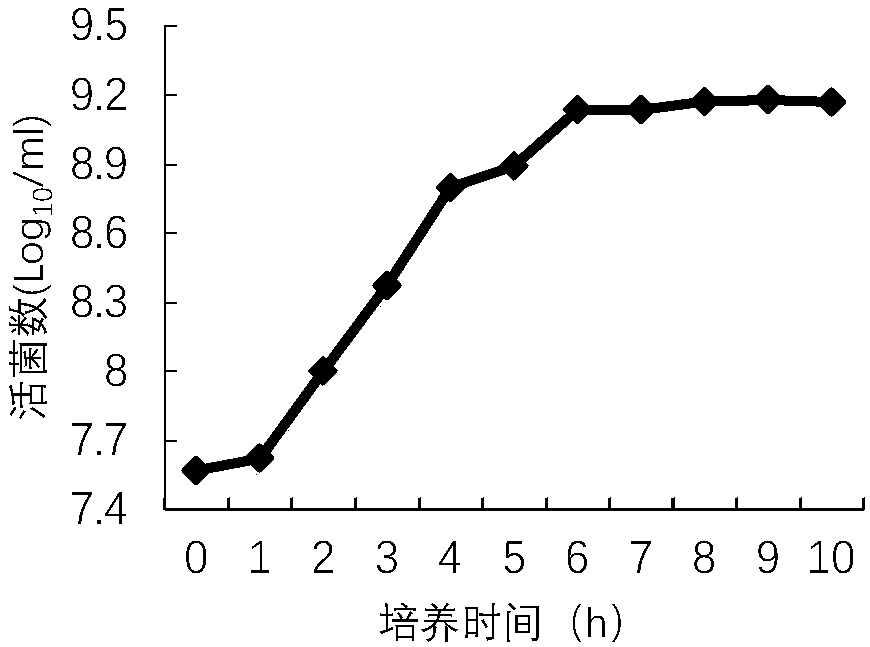
[0061] 1. Take the frozen bacterial liquid of the P2 generation DCP1 strain, thaw at room temperature, inoculate BHI liquid medium at a volume ratio of 1% to 10%, and culture at 37°C and 150rpm / min for 5 to 18 hours with shaking. The above-mentioned activated bacterial solution was inoculated into BHI medium at a ratio of 1% to 10%, cultured at 37°C and 150 rpm / min for 5 to 18 hours with shaking, and tested according to the appendix of the current Veterinary Pharmacopoeia of the People's Republic of China. The number of viable bacteria was calculated by counting viable bacteria.
[0062] 2. Add 37-40% formaldehyde solution to the above cultured bacterial solution and make the formaldehyde concentration 0.2%-0.5% (volume ratio, 37°C, 150rpm...
Embodiment 3
[0068] Embodiment 3, the inspection of vaccine
[0069] 1. Dosage Form
[0070] Get a 1ml disposable pipette, absorb a small amount of each vaccine prepared in Example 2 and drop it on the cold water surface installed on a 90mm disposable plate, except for the first drop, it does not spread.
[0071] 2. The stability of the vaccine
[0072] Take 10ml of each vaccine prepared in Example 2 and add it to a 10ml centrifuge tube, centrifuge at 3500r / min for 15 minutes, and the water phase precipitated at the bottom of the tube is not more than 0.5ml. The vaccines prepared in Example 2 were stored at 2-8°C for 24 months, at 37°C for 1 month or at 25°C for 3 months without discoloration, delamination and demulsification.
[0073] 3. Vaccine sterility test
[0074] 1. Take the above-mentioned antigens and carry out the sterility test according to the method of "The Veterinary Pharmacopoeia of the People's Republic of China", and the result is no bacterial growth.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com