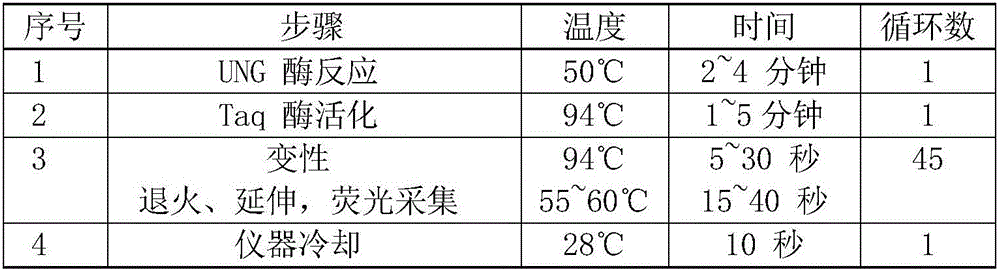
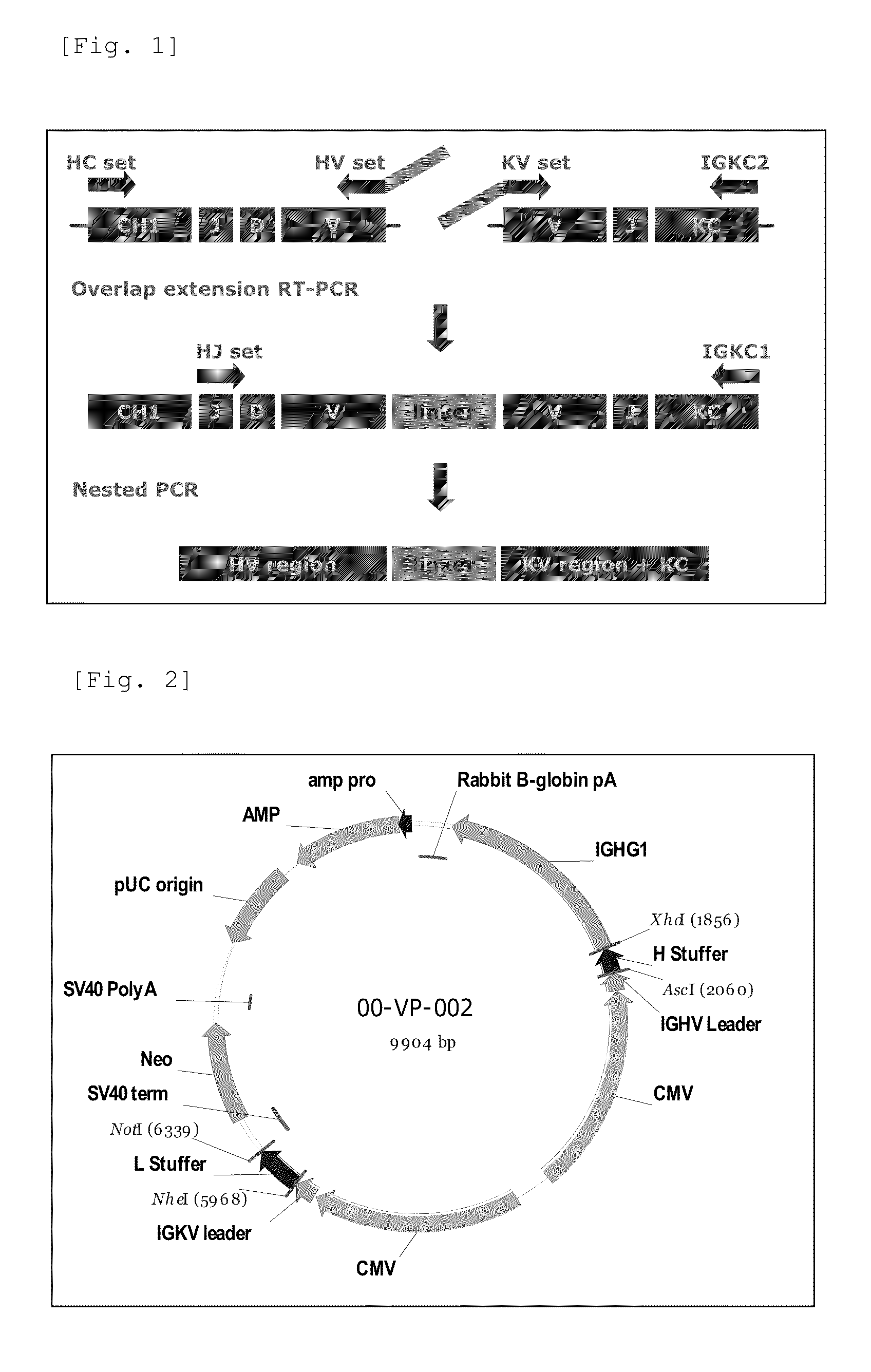
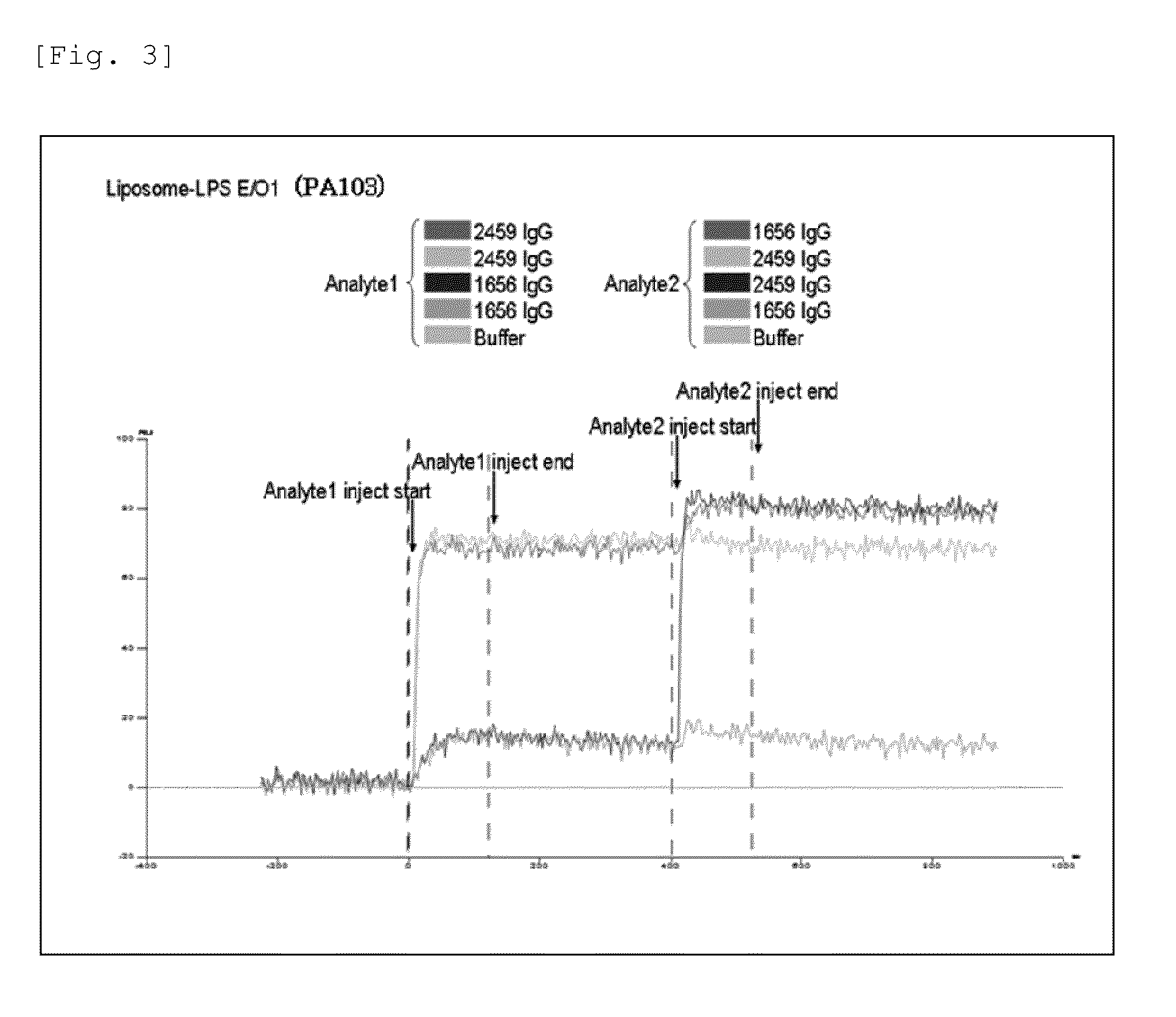
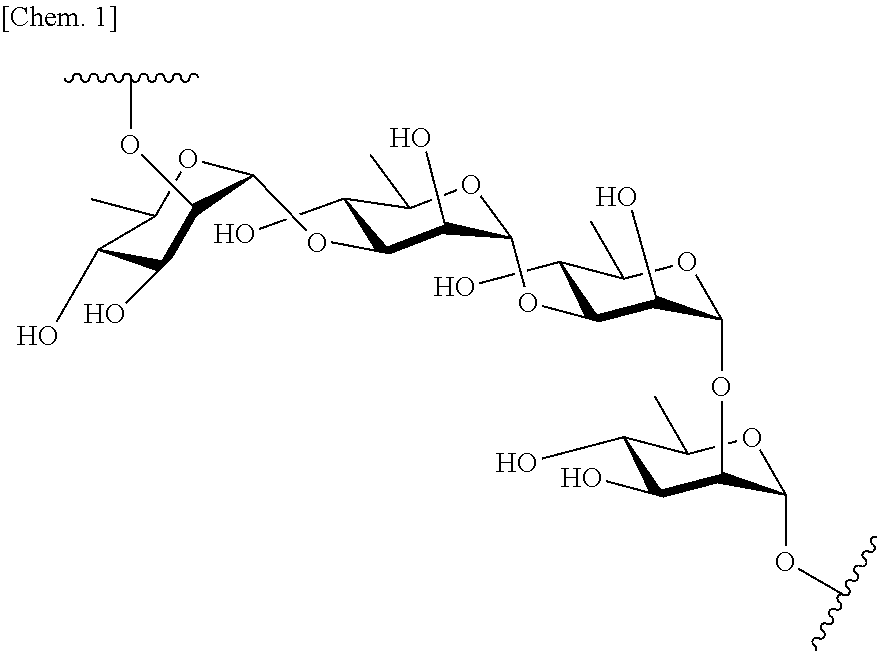
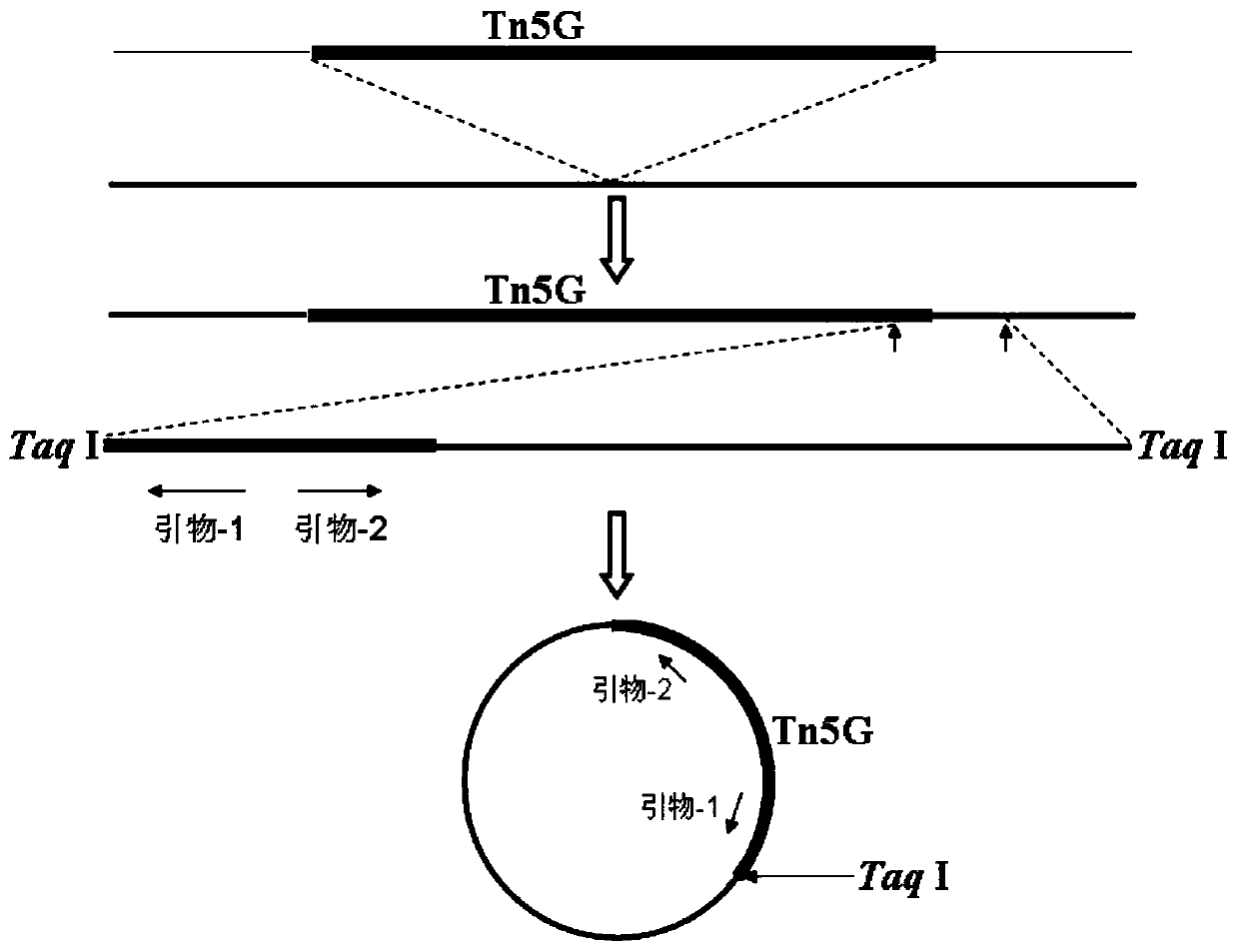
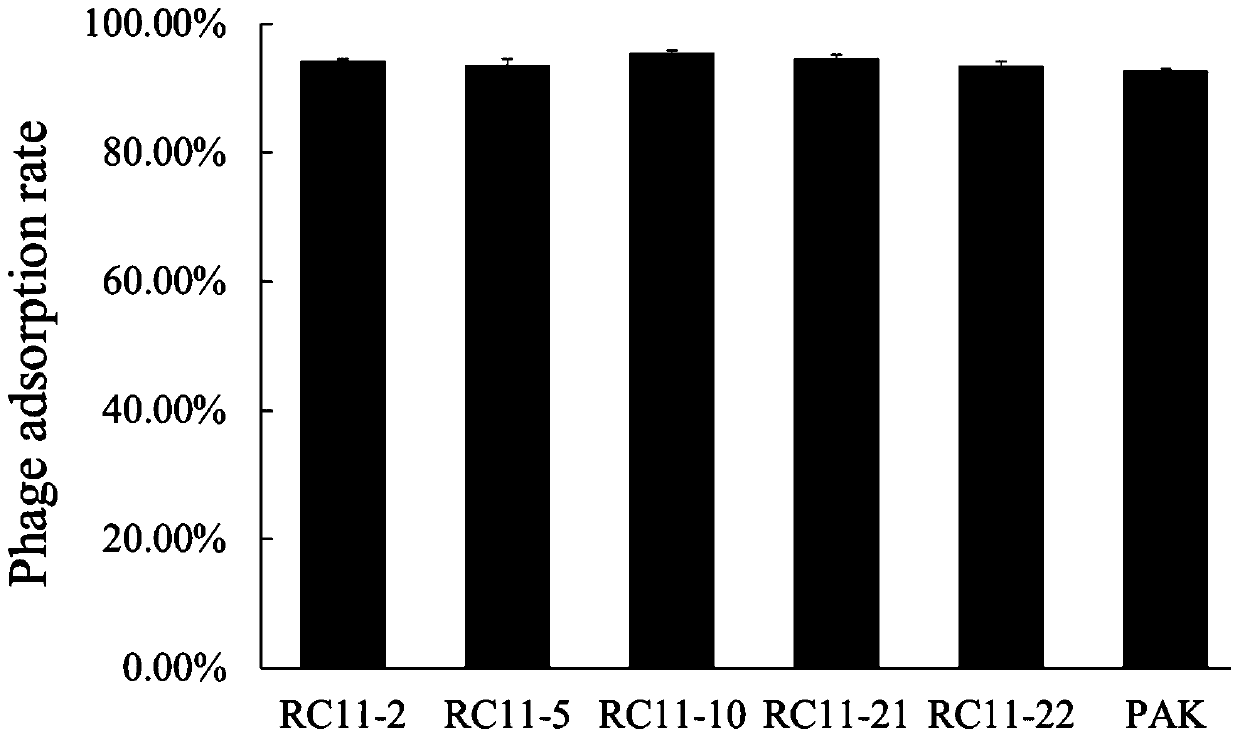
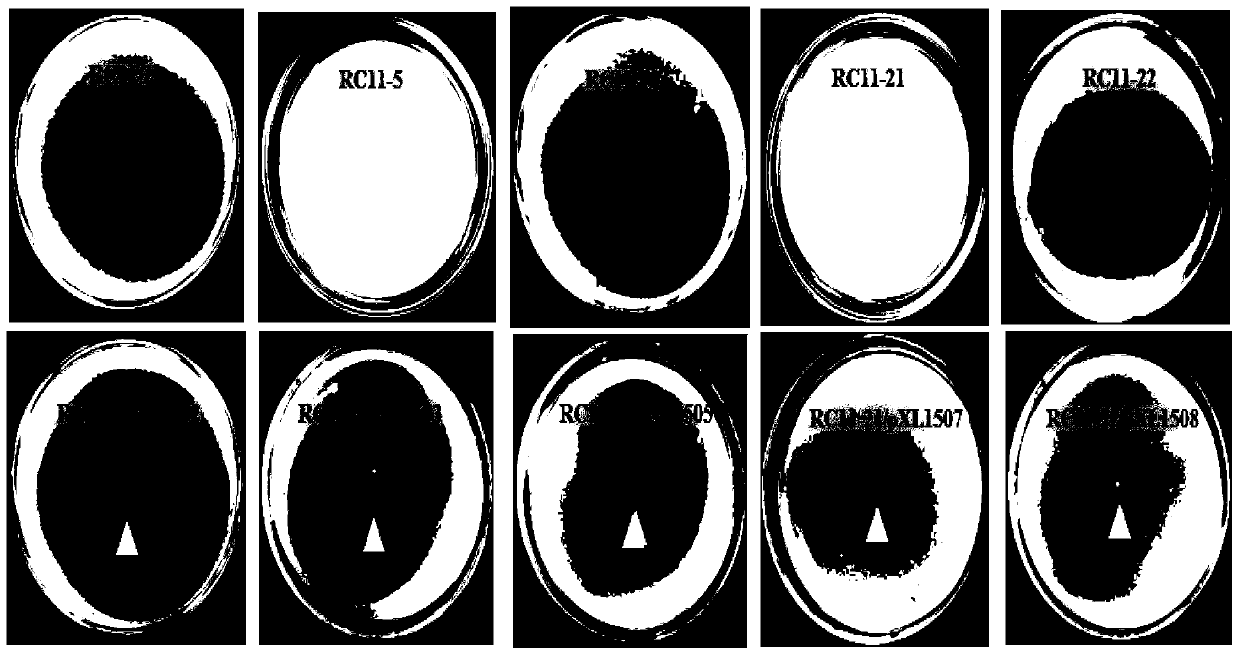
Patents
Literature
32 results about "Pseudomonas aeruginosa DNA" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pseudomonas aeruginosa and application of pseudomonas aeruginosa in aspect of degrading aflatoxin
ActiveCN103710292AEfficient degradationBacteriaMicroorganism based processesMicrobiologyAflatoxin degradation
The invention discloses pseudomonas aeruginosa and application of the pseudomonas aeruginosa in the aspect of degrading aflatoxin. Preservation No. of the pseudomonas aeruginosa disclosed by the invention is CGMCC No. 8511. The pseudomonas aeruginosa disclosed by the invention can degrade aflatoxin effectively; the bacterium, as a biomaterial for degrading the aflatoxin, has excellent application prospects in development of new biodegradable bacterium or biodegradable sterile preparation.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Primer, kit and method for detecting pseudomonas aeruginosa and clostridium perfringens
InactiveCN107513561AReasonable compositionReasonable ratioMicrobiological testing/measurementMicroorganism based processesBacillus perfringensPseudomonas aeruginosa DNA
The invention discloses a primer, a kit and a method for detecting Pseudomonas aeruginosa and Clostridium perfringens. The primer sequence and probe sequence are respectively: upstream primer P.AF, downstream primer P.AR, probe P .AP, upstream primer C.PF, downstream primer C.PR:, probe C.PP. The purpose of the present invention is to overcome the deficiencies in the prior art, to provide a primer with reasonable components and ratio, easy to use, fast and accurate detection, suitable for double ddPCR detection of Pseudomonas aeruginosa and gas production in water The test kit of Clostridium perfringens; Another purpose of the present invention is to provide a kind of method that adopts above-mentioned test kit to detect Pseudomonas aeruginosa and Clostridium perfringens in water, and this method is easy and simple to operate, fast, and cost is low, and detection result precise.
Owner:INSPECTION AND QUARANTINE TECHNOLOGY CENTER ZHONGSHAN ENTRY EXIT INSPECTION AND QUARANTINE
Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method
InactiveCN106520984AQuick destructionStrong specificityMicrobiological testing/measurementMicroorganism based processesForward primerPositive control
The invention discloses a Pseudomonas aeruginosa nucleic acid fluorescent PCR (polymerase chain reaction) detection kit and detection method. The detection kit comprises a PCR reaction solution, an enzyme mixed solution, a positive control, a negative control and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, primers for target polynucleotide amplification and probes for target polynucleotide detection; the primers comprise a forward primer and a reverse primer. Or, the detection kit comprises a PCR reaction solution, an enzyme mixed solution and an internal standard, wherein the PCR reaction solution comprises a PCR buffer solution, nucleic acid releaser, deoxyribonucleoside triphosphate, a forward primer for target polynucleotide amplification, a reverse primer for target polynucleotide amplification and probes for target polynucleotide detection. The Pseudomonas aeruginosa nucleic acid fluorescent PCR detection kit shown in the embodiment of the invention with the advantages of no need of nucleic acid extraction, high detection sensitivity, wide detection range and high detection accuracy can quickly and accurately detect Pseudomonas aeruginosa DNA (deoxyribonucleic acid) in plasma, urine and other samples.
Owner:SANSURE BIOTECH INC
Multiple PCR detection primers of escherichia coli, pseudomonas aeruginosa and staphylococcus aureus, kit and detection method
InactiveCN106399568AAccurate detectionEfficient detection methodMicrobiological testing/measurementMicroorganism based processesEscherichia coliStaphylococcus cohnii
The invention discloses multiple PCR detection primers of escherichia coli, pseudomonas aeruginosa and staphylococcus aureus, a kit and a detection method. Specific primers are designed by means of combined utilization of escherichia coli alkaline phosphatase gene, pseudomonas aeruginosa outer membrance proteins gene and staphylococcus aureus heat-resistant nuclease gene sequences, multiple PCR detection is conducted on a sample by means of the specific primers according to the method, and specific detection of the escherichia coli, the pseudomonas aeruginosa and the staphylococcus aureus in the sample can be achieved at a time; the detection time is short, operation is easy and convenient, the detection efficiency can be effectively improved, the requirement of rapid detection is met, the sensitivity is high, the specificity is high, and the multiple PCR detection primers of the escherichia coli, the pseudomonas aeruginosa and the staphylococcus aureus, the kit and the detection method can be applied to microbiological detection work of cosmetics.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
Verdigris pseudomonas aeruginosa strain denitrified under different dissolved oxygen conditions and application thereof
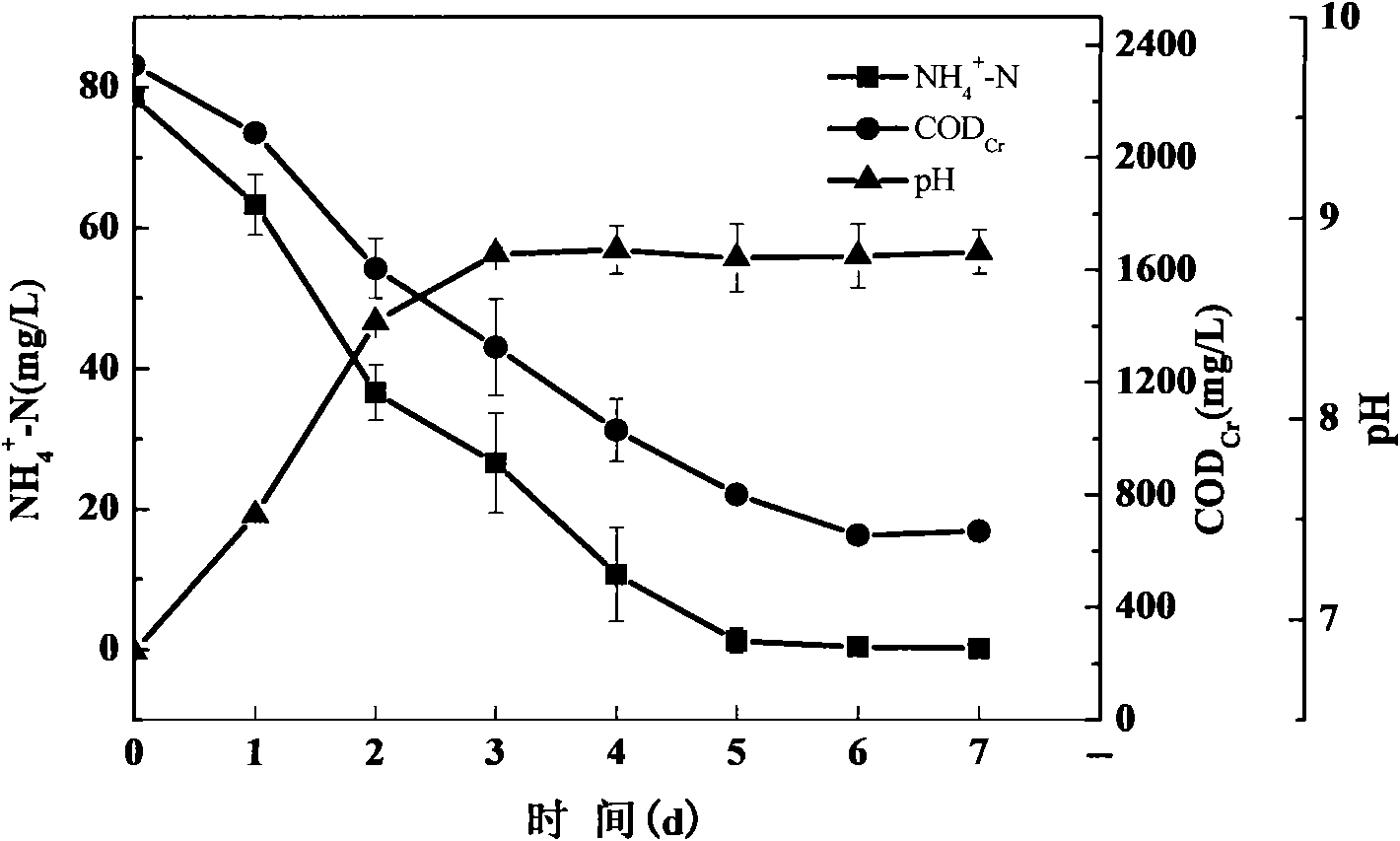
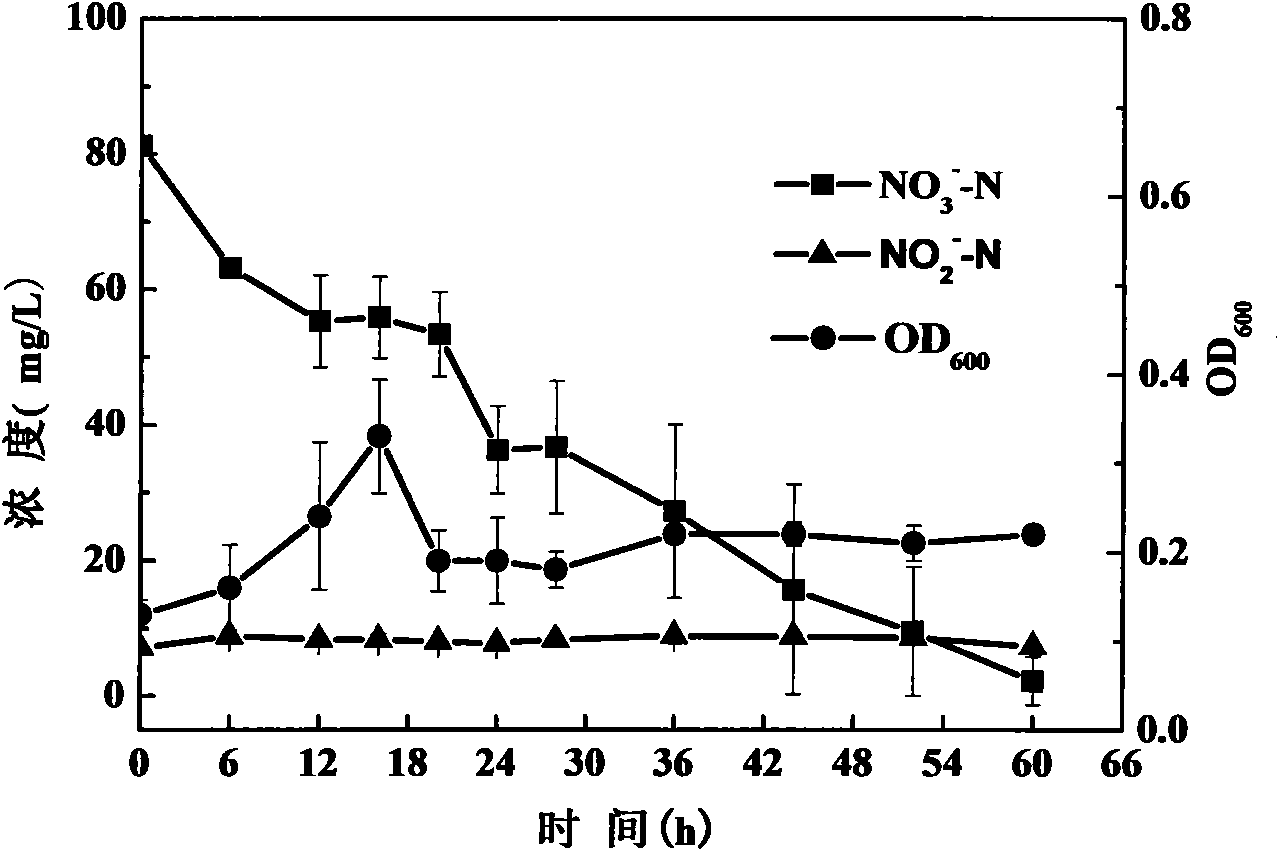
The invention discloses a verdigris pseudomonas aeruginosa strain denitrified under different dissolved oxygen conditions and the application thereof and also discloses a microbiological bacterial inoculum for biological denitrification. The pseudomonas aeruginosa HD4-2 provided by the invention was preserved in CGMCC (China General Microbiological Collection Center) on Jan. 21 in 2010 and has a preservation number of CGMCC No.3602. The strain can remove ammonia nitrogen under anoxic, micro-aerobic and aerobic conditions with the removal rate over 95%, can independently finish the whole process of biological denitrification without the accumulation of nitrite nitrogen and nitric nitrogen and has higher potential application values in practice.
Owner:PEKING UNIV
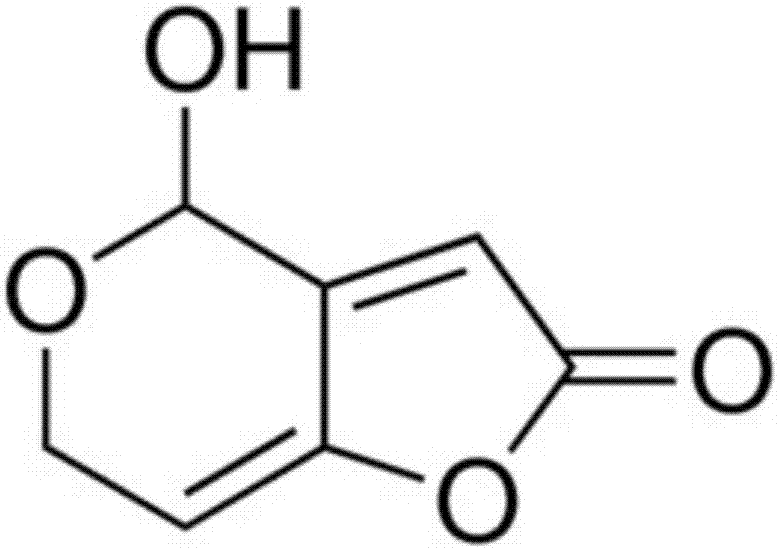
Application of pseudomonas aeruginosa to patulin degradation
The invention relates to the field of microorganisms, and specifically discloses a new application of pseudomonas aeruginosa, namely the new application of the pseudomonas aeruginosa to patulin degradation. According to the invention, it is proved by experiments that the pseudomonas aeruginosa can degrade the patulin effectively. The pseudomonas aeruginosa has a good application prospect in developing both of new biodegradation microbial preparations and new biodegradation sterile preparations.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
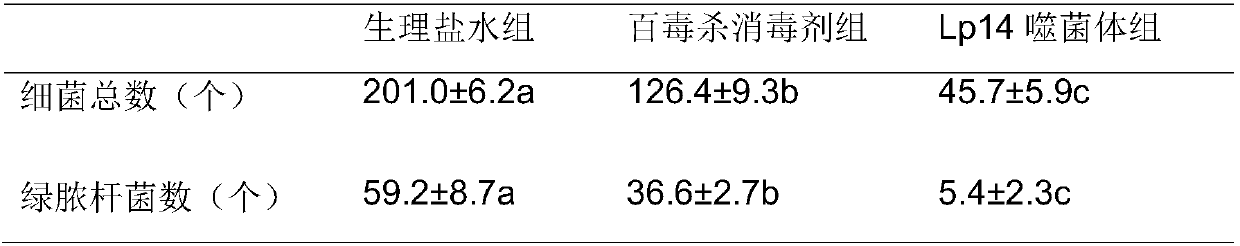
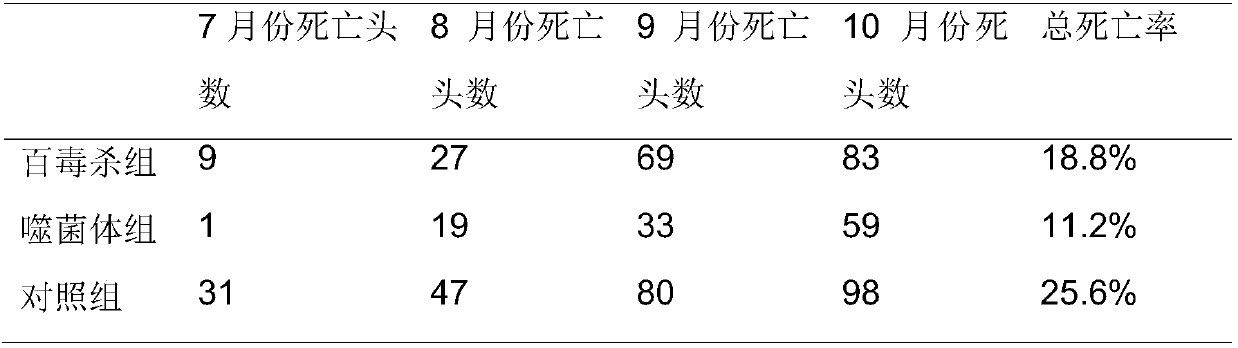
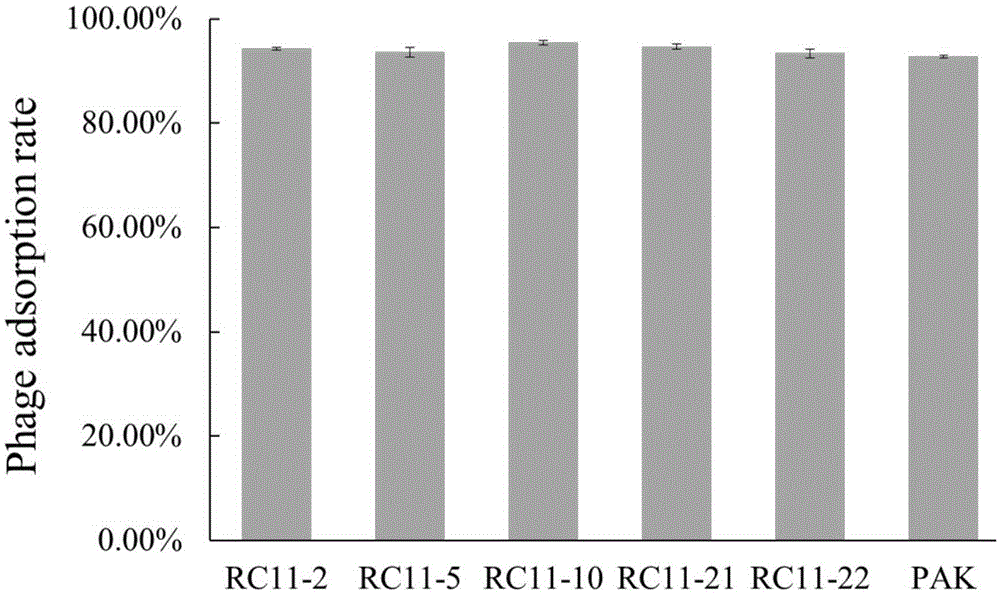
Wide-lysis-spectrum pseudomonas aeruginosa phage and sterilization application thereof
The invention belongs to the field of bioengineering and provides a phage with environment sterilization capacity and application thereof. A phage monomer is the pseudomonas aeruginosa phage, the Latin name is P.aeruginosaphage, the phage monomer is named as Lp14 and has broad-spectrum sterilization capacity on pseudomonas aeruginosa, the phage Lp14 is preserved in the general microbiological center of the China microbe preservation management committee, the preservation date is thirtieth, March, 2017, and the preservation number is CGMCC No.13791. The phage has a high splitting function on pseudomonas aeruginosa, and a phage source is provided for industrial phage production, disinfection and sterilization; the phage can also be used as a safe biological disinfector and used for sterilizing the environment of a mink field, and accordingly the hemorrhagic pneumonia caused by pseudomonas aeruginosa during mink breeding is reduced. The phage can be applied to industrial production, subjected to specific amplification through host pseudomonas aeruginosa, used as the biological disinfector and applied to sterilization for the environment of the mink field.
Owner:QINGDAO PHAGEPHARM BIO TECH CO LTD +1
Phage Therapy Against Pseudomonas Aeruginosa
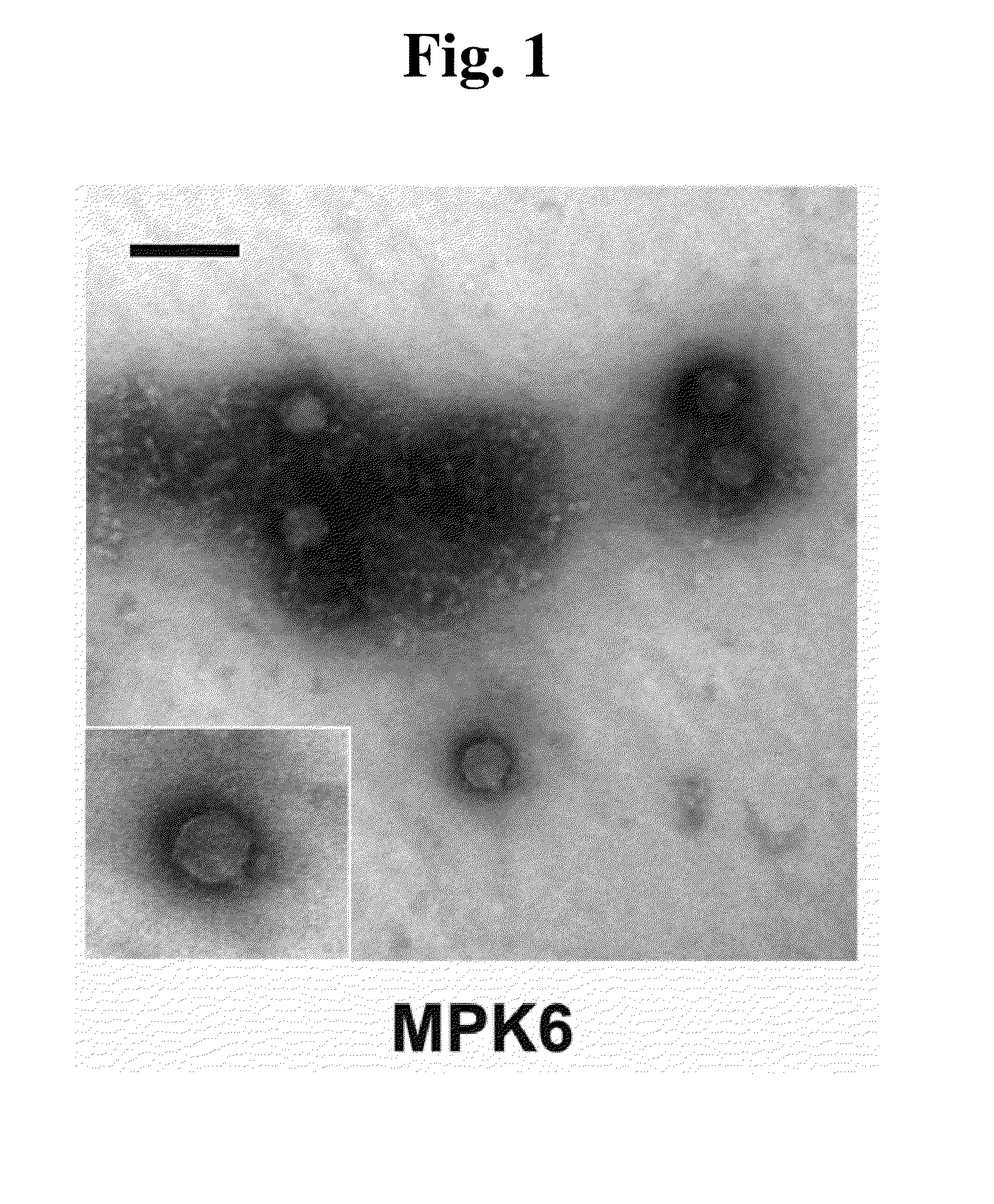
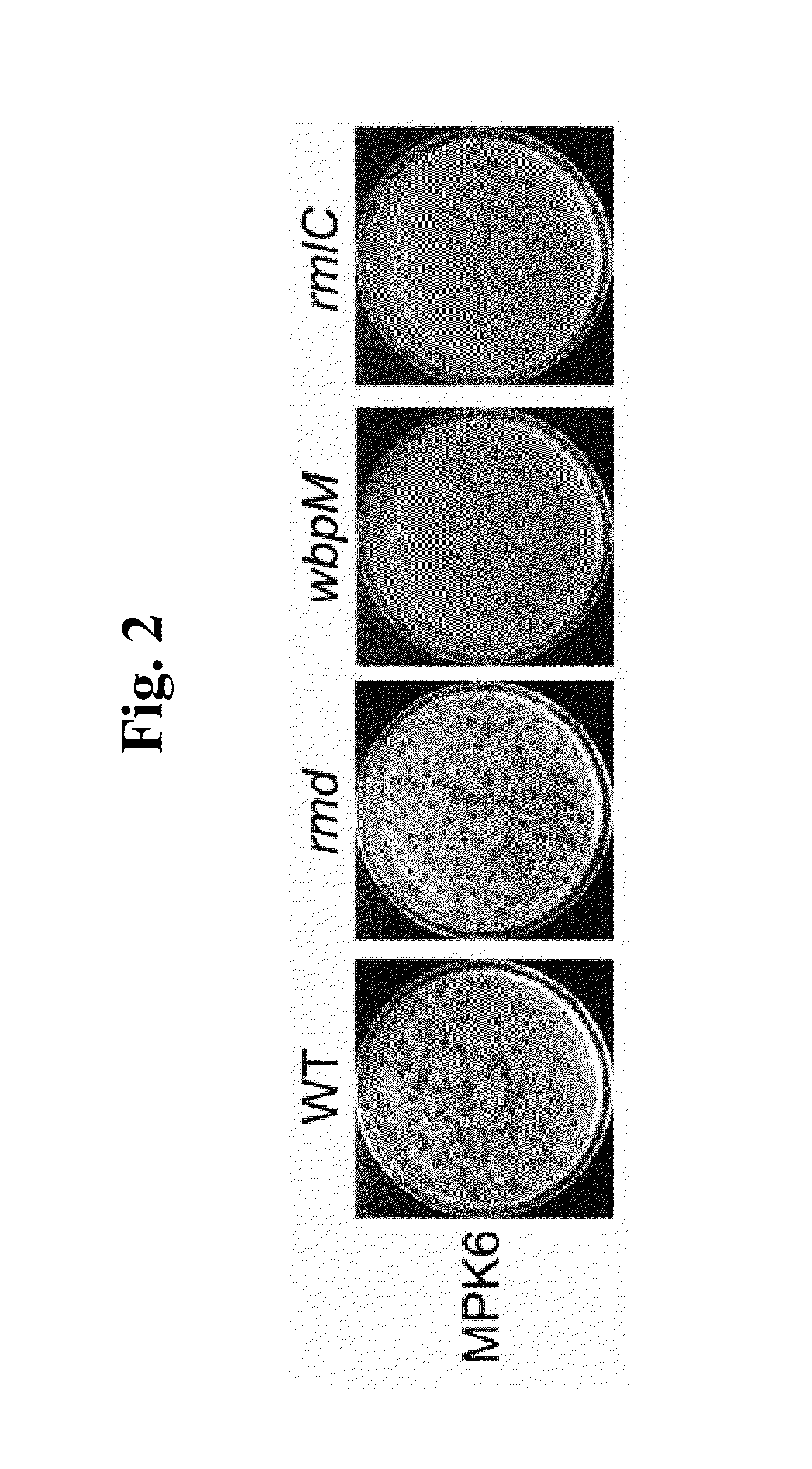
This invention relates to a bacteriophage MPK6 (deposit number: KCCM 11044P) having a lytic activity to Pseudomonas aeruginosa, or a progeny bacteriophage thereof having a RFLP (Restriction fragment length polymorphism) DNA profile substantially equivalent to the bacteriophage MPK6. The present invention provides a bacteriophage MPK6 or a progeny bacteriophage thereof capable of treating a Pseudomonas aeruginosa infection disease, and suggests an anti-bacterial activity of MPK6 and its progeny bacteriophage using a mammalian and non-mammalian infection model. According to the present invention, the present bacteriophage MPK6 or progeny bacteriophage thereof represents very effective efficacy on treatment of P. aeruginosa-induced peritonitis-sepsis.
Owner:IND UNIV COOP FOUND SOGANG UNIV
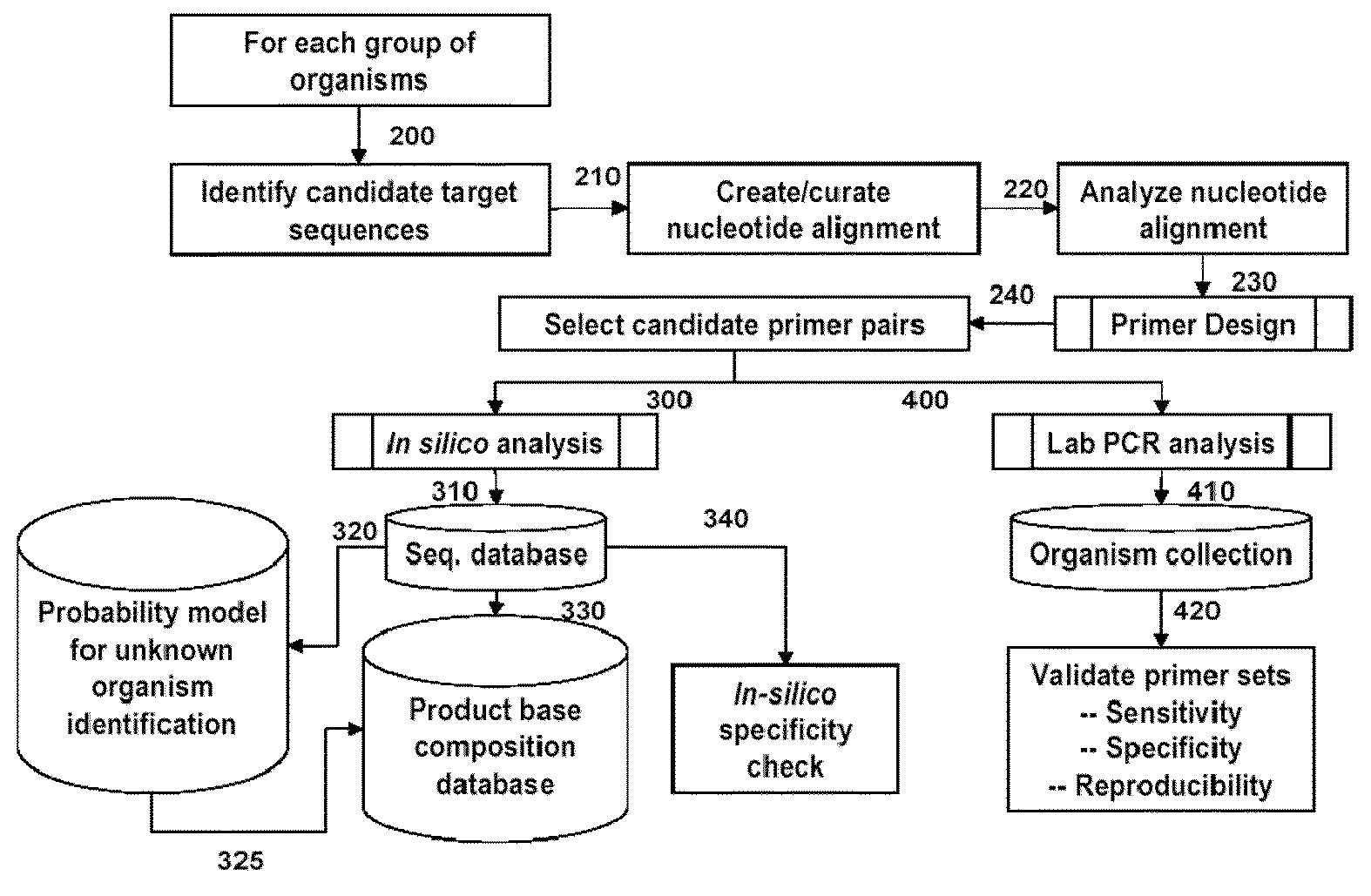
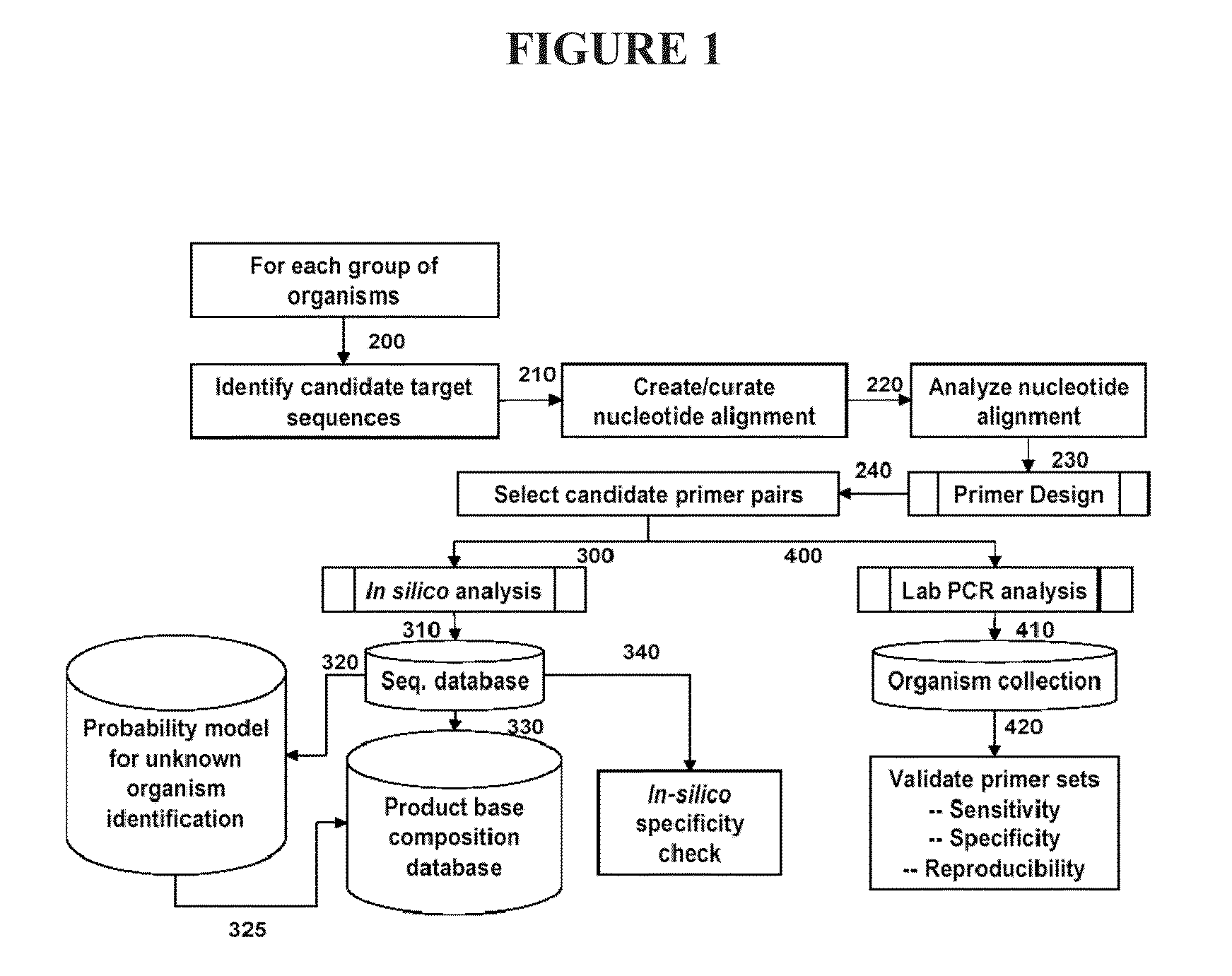
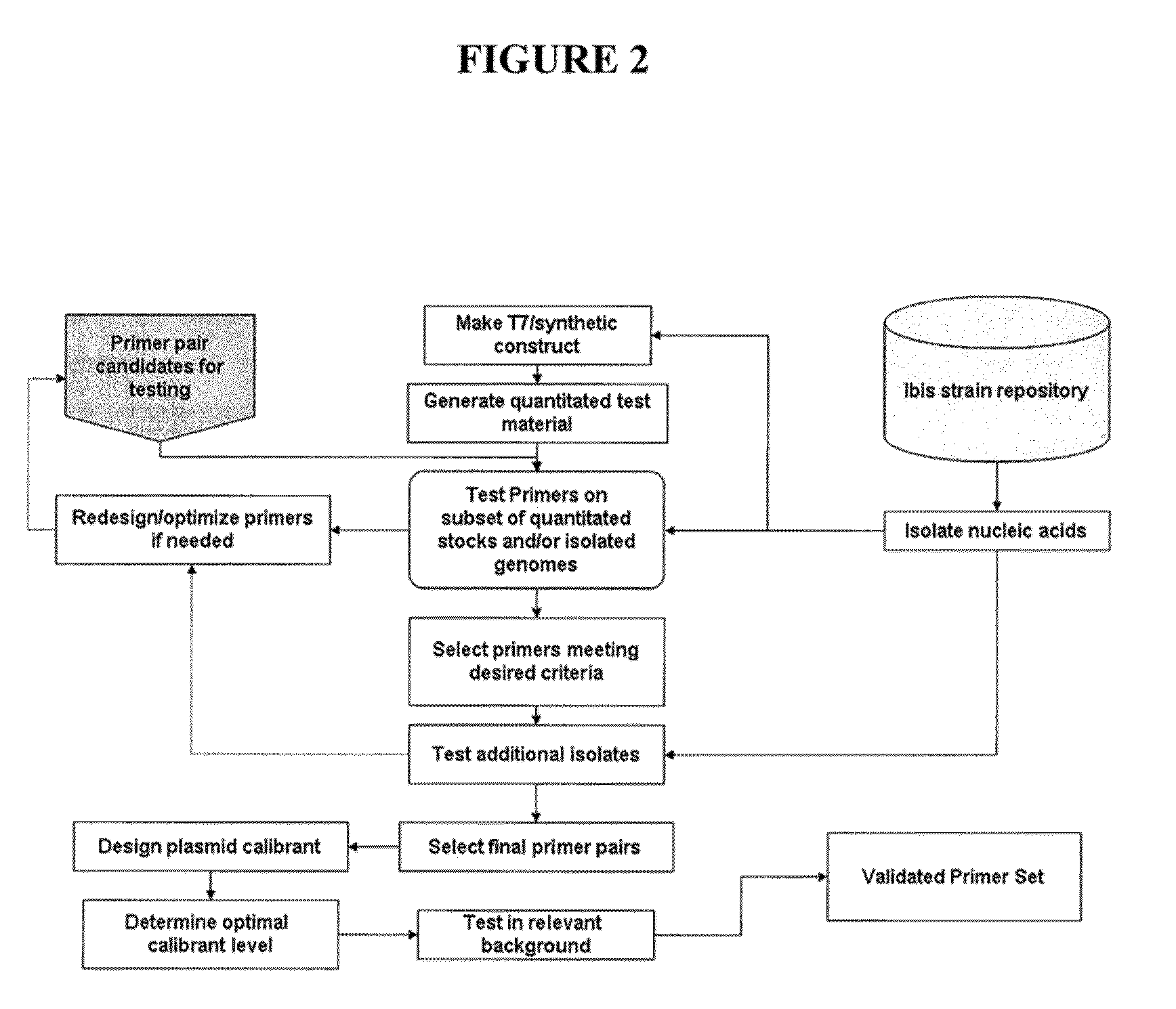
Compositions for use in identification of pseudomonas aeruginosa
The present invention relates generally to identification of Pseudomonas aeruginosa bacteria or strains of Pseudomonas aeruginosa, and provides methods, compositions and kits useful for this purpose when combined, for example, with molecular mass or base composition analysis.
Owner:IBIS BIOSCI
Primers for detecting Pseudomonas aeruginosa through loop-mediated isothermal amplification technology, and kit
InactiveCN107164497AHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationSpecific detectionLoop-mediated isothermal amplification
The invention discloses a set of primers for detecting Pseudomonas aeruginosa by a loop-mediated isothermal amplification technique and a rapid diagnosis kit for Pseudomonas aeruginosa gene. Prior art Chinese patent CN 102134601 A discloses a loop-mediated isothermal amplification detection primer, detection method and detection kit for Pseudomonas aeruginosa. This invention designs and screens the ecfX gene of Pseudomonas aeruginosa A set of specific detection primers, a detection kit containing the detection primers and the detection of LAMP by using the detection kit. Compared with the prior art, the present invention designs a group of new primers based on the ecfX gene. Through the comparison experiment of two groups of primers, the primers of the present invention have better sensitivity and high sensitivity.
Owner:赵吉光
Method for preparing proteus mirabilis-staphylococcus aureus-pseudomonas aeruginosa adsorption combined vaccine
InactiveCN105709218ARich varietySimple processAntibacterial agentsBacterial antigen ingredientsStaphylococcus cohniiUltrafiltration
The invention discloses a method for preparing a proteus mirabilis-staphylococcus aureus-pseudomonas aeruginosa adsorption combined vaccine. The method includes the steps that firstly, a proteus mirabilis culture solution is subjected to in-situ digestion, high-speed centrifugation, pre-filtering, ultrafiltration and precipitation, and a proper proteus mirabilis cell membrane soluble antigen raw solution is obtained; secondly, a staphylococcus aureus bacterium suspension is subjected to centrifugation, smashing, re-centrifugation, filtering, precipitation, enzymolysis and dialysis, and a proper staphylococcus aureus cytoplast antigen raw solution is obtained; thirdly, a staphylococcus aureus and pseudomonas aeruginosa culture solution is subjected to centrifugation and pre-filtering, formalin is added for detoxification, then purification is conducted, and a proper inactivated staphylococcus aureus and pseudomonas aeruginosa toxin raw solution is obtained. The vaccine is used for preventing burn, scalding and infection caused by one or more of conditioned pathogens including proteus mirabilis, staphylococcus aureus and pseudomonas aeruginosa before and after operations in an intramuscular deep injection mode.
Owner:LIAONING CHENGDA BIOTECH
Antibody against serotype e lipopolysaccharide of pseudomonas aeruginosa
InactiveUS20130004499A1High antibacterial activityEffective treatmentAntibacterial agentsAnimal cellsPulmonary infectionSerotype
Provided is a novel antibody having an excellent antibacterial activity against P. aeruginosa. By using plasmablasts obtained from cystic fibrosis patients with chronic P. aeruginosa pulmonary infection as starting materials, antibodies which bind to LPS of a P. aeruginosa strain of serotype E and which have excellent antibacterial activities in vitro and in vivo were successfully obtained.
Owner:MEIJI SEIKA PHARMA CO LTD +1
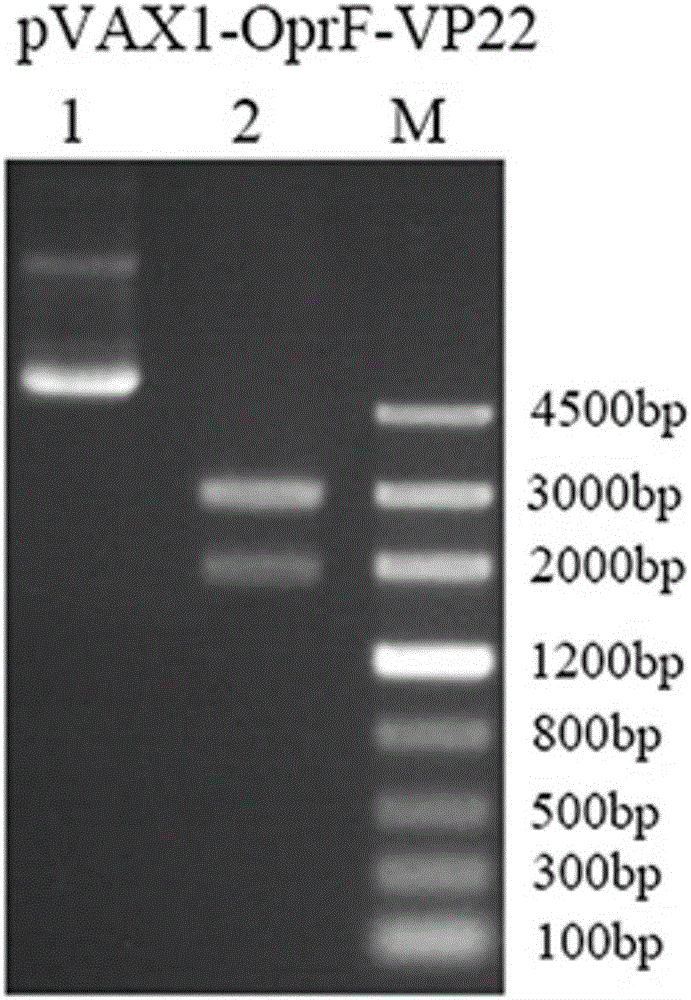
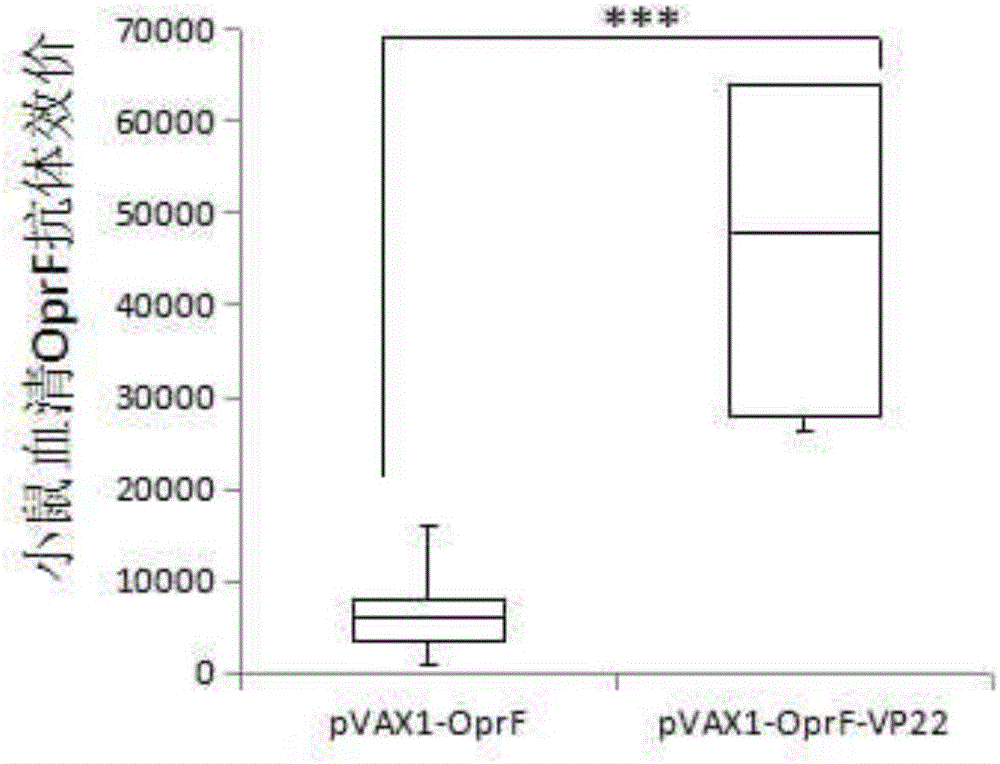
Pseudomonas aeruginosa gene and DNA vaccine thereof
ActiveCN105713916AEnhance humoral immunityHigh antibody titerPolypeptide with localisation/targeting motifAntibacterial agentsMicrobiologyGene
The invention discloses a pseudomonas aeruginosa OprF-VP22 gene, the application of the gene for making vaccines for controlling pseudomonas aeruginosa infection, and a pseudomonas aeruginosa DNA vaccine containing the gene.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
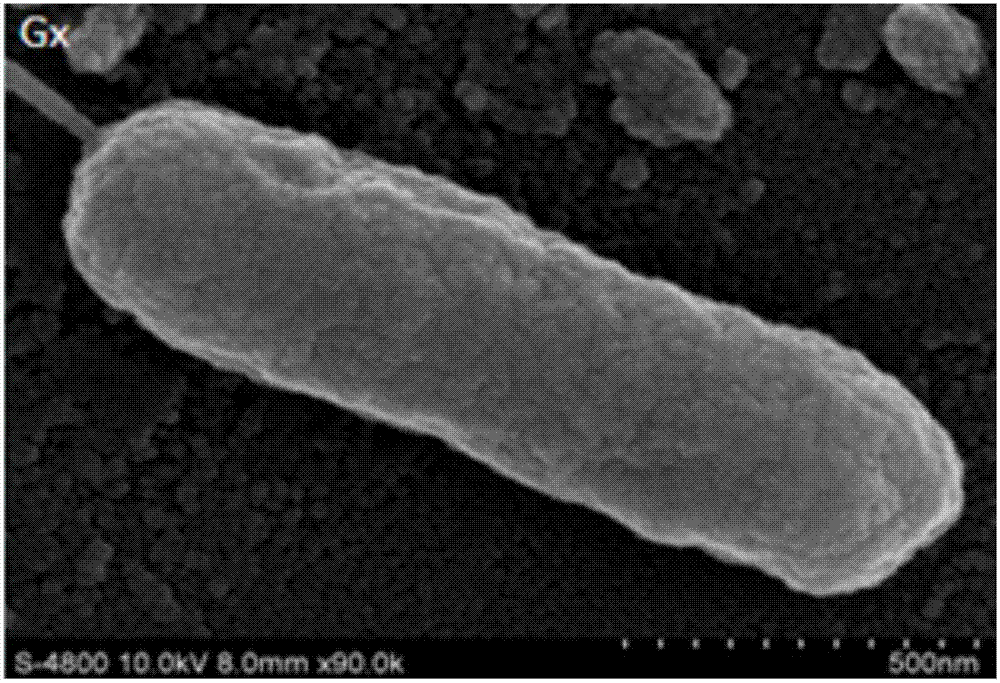
Pseudomonas aeruginosa and applications thereof
ActiveCN107099470ALow viscosityGood emulsifying effectBacteriaMicroorganism based processesMicrobial enhanced oil recoveryGasoline
The invention discloses pseudomonas aeruginosa and particularly discloses pseudomonas aeruginosa Gx preserved in CGMCC (China General Microbiological Culture Collection Center) with the preservation number of CGMCC NO. M2016790 on December 30th 2016. The invention also discloses applications of the pseudomonas aeruginosa Gx to asphalt degradation and transformation, colloid degradation and transformation and microbial enhanced oil recovery. The pseudomonas aeruginosa Gx disclosed by the invention has a strong degradation ability on colloid and unknown macromolecule components in asphalt and crude oil, strong surface active material synthesizing ability and acid producing ability and large oil displacing potential in the microbial enhanced oil recovery of high-asphalt content heavy oil reservoirs; and therefore, the pseudomonas aeruginosa Gx is capable of remarkably reducing the adhesion of the crude oil and increasing the gasoline and diesel oil yield in crude oil refining.
Owner:陕西博秦生物工程有限公司 +1
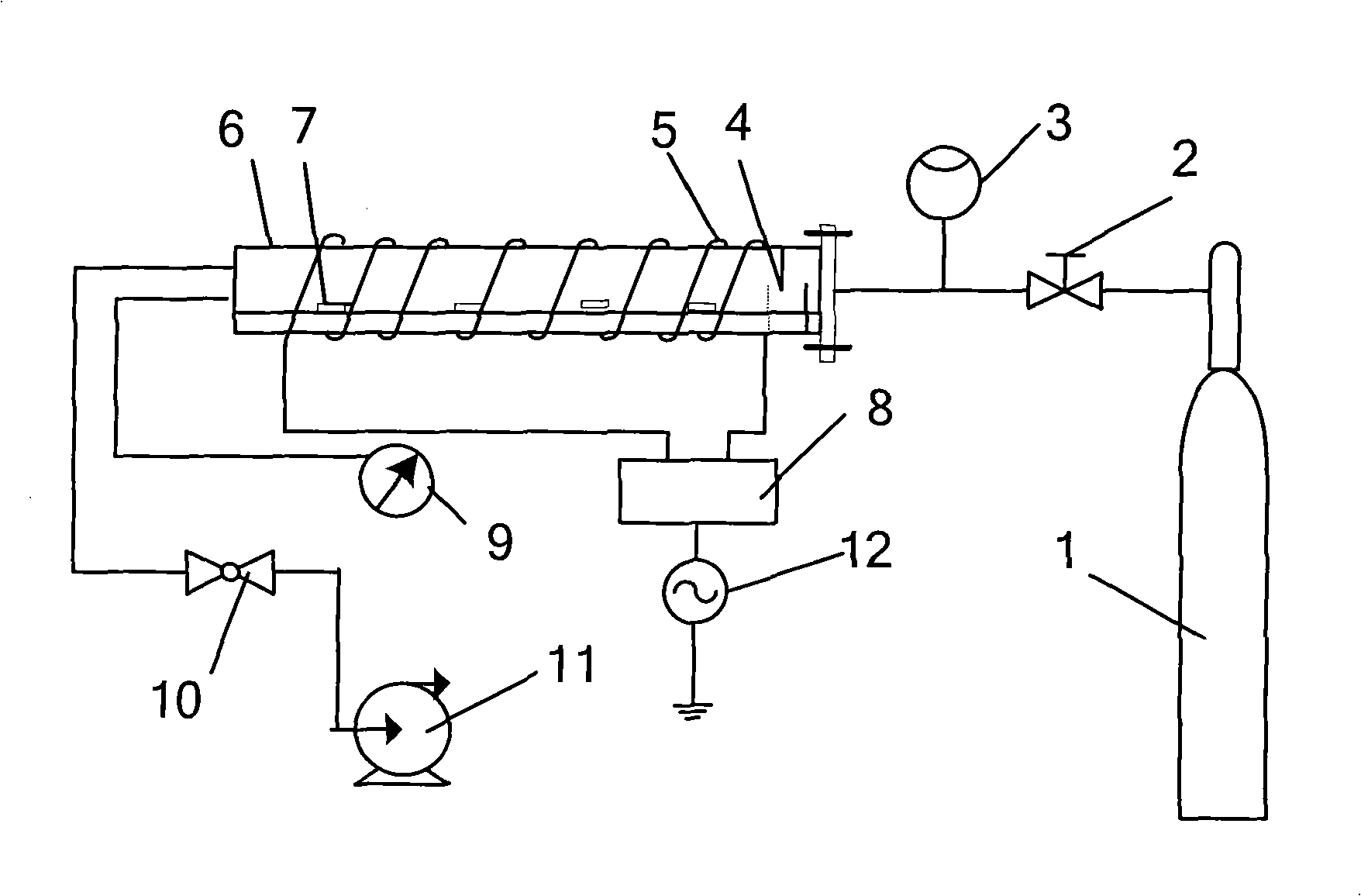
Method for killing pseudomonas aeruginosa
InactiveCN101537197AQuick killEfficient killingLavatory sanitoryDisinfectionLow temperature plasmaToxicology
The invention relates to the field of disinfection and sterilization, which discloses a method for killing pseudomonas aeruginosa. The method is achieved only by putting a carrier infected with the pseudomonas aeruginosa in plasma environment at a low temperature of between 10 DEG C and 50 DEG C for killing the pseudomonas aeruginosa for 30-100 seconds, wherein the low-temperature plasma environment is generated by a radio frequency plasma discharge device.
Owner:XI AN JIAOTONG UNIV
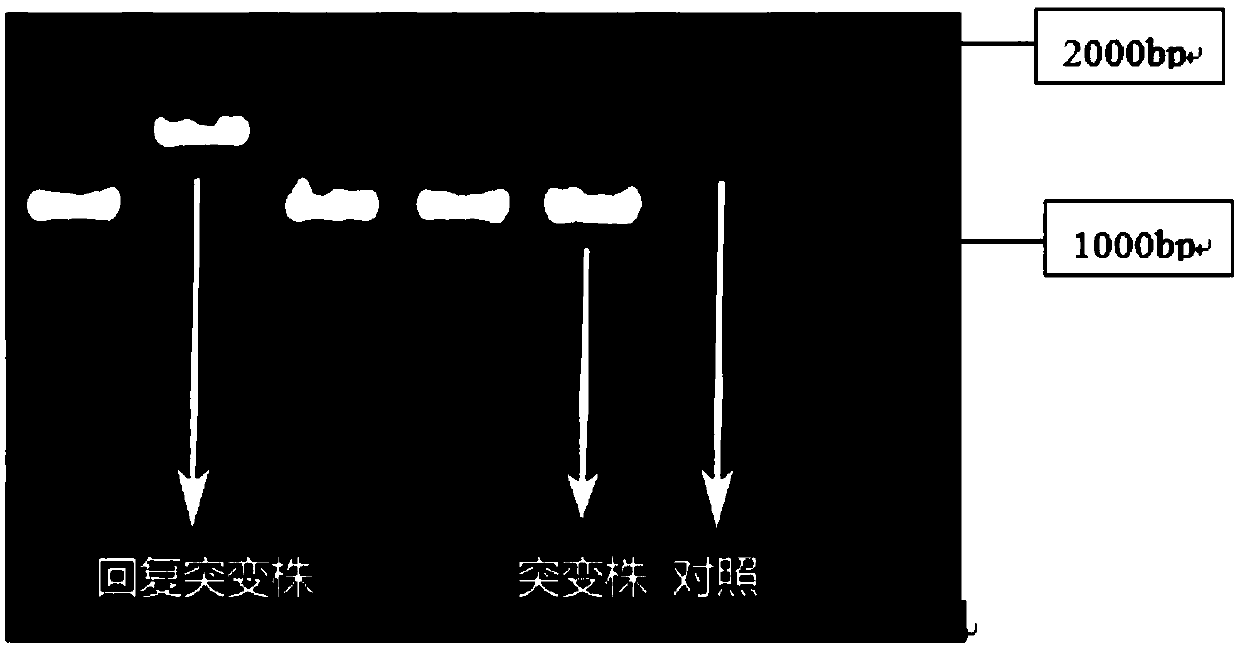
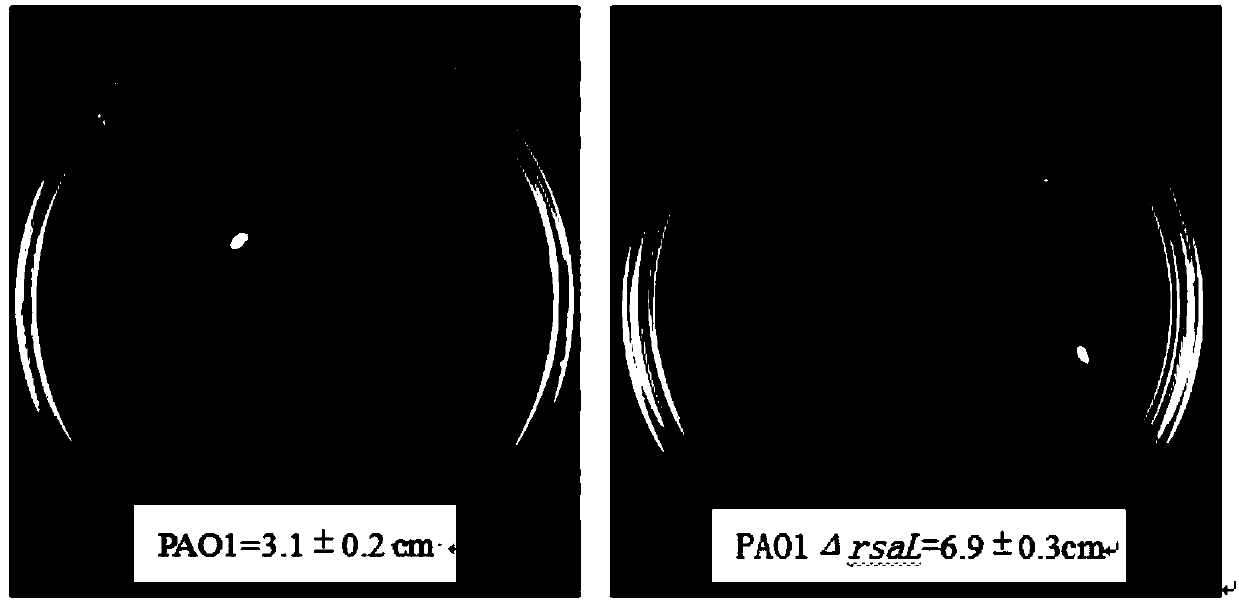
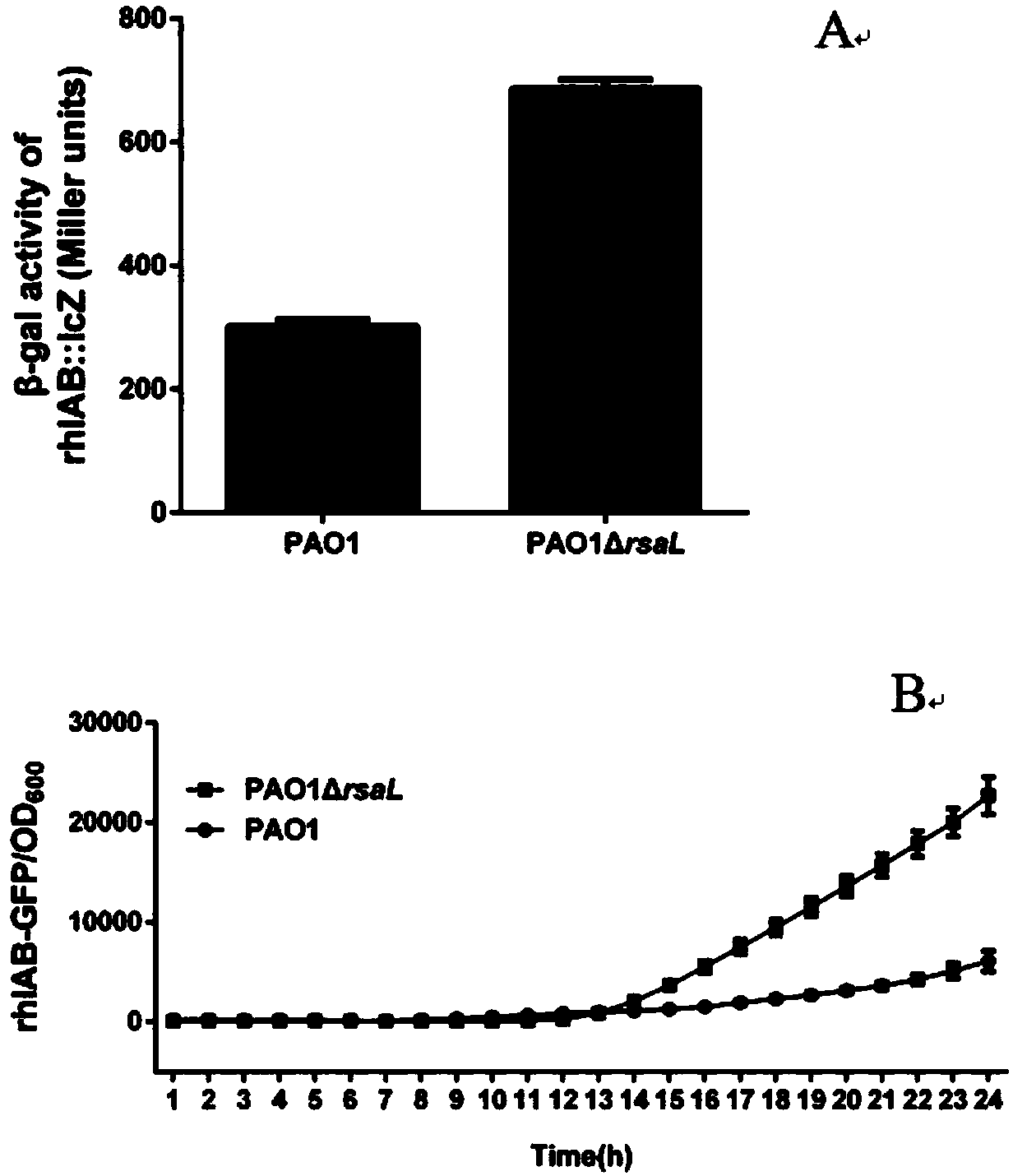
Application for suppressing expression of gene rsaL in pseudomonas aeruginosa and pseudomonas aeruginosa with suppressed expression of gene rsaL
InactiveCN107828796AIncrease productionImprove performanceBacteriaMicroorganism based processesPseudomonas cichoriiAntibiotic Y
The invention relates to the field of biology and particularly relates to an application for suppressing expression of a gene rsaL in pseudomonas aeruginosa and the pseudomonas aeruginosa with suppressed expression of the gene rsaL. The application is improvement of the transcriptional levels of rhamnolipid-synthesizing genes rhlA and rhlB, the rhamnolipid-synthesizing capability and the swarmingmoving capability of the pseudomonas aeruginosa. According to the transcriptional levels of the rhamnolipid-synthesizing genes rhlA and rhlB and synthesis of total rhamnolipid, dirhamnolipid Rha-Rha-C10-C10 and monorhamnolipid Rha-C10-C10 of the strain provided by the invention, the obtained yield is higher, the property is more stable and the problem of mutation restoration in the process of physicochemical mutagenesis is solved. In addition, the swarming moving capability of the rhamnolipid strain provided by the invention is enhanced. Simultaneously, the strain constructed by the inventionhas the beneficial effects that antibiotics do not need to be added in the production process of rhamnolipid, so that the benefit for the following separation purification is achieved.
Owner:DONGGUAN UNIV OF TECH
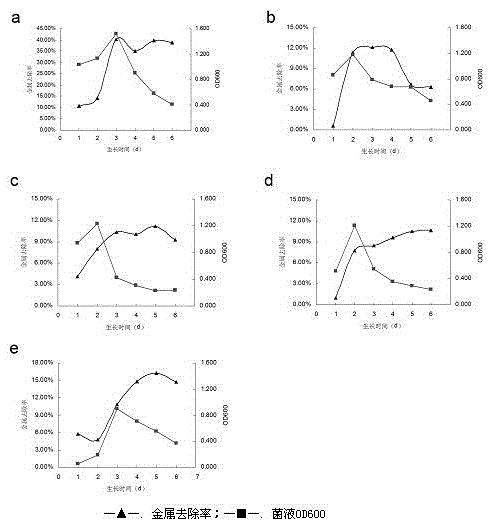
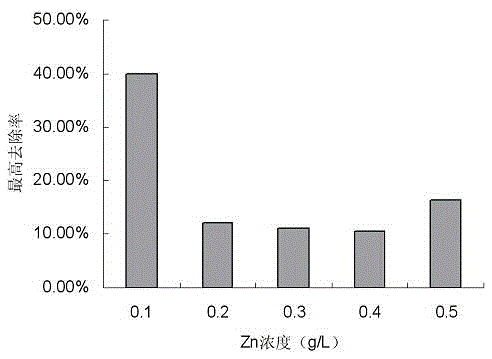
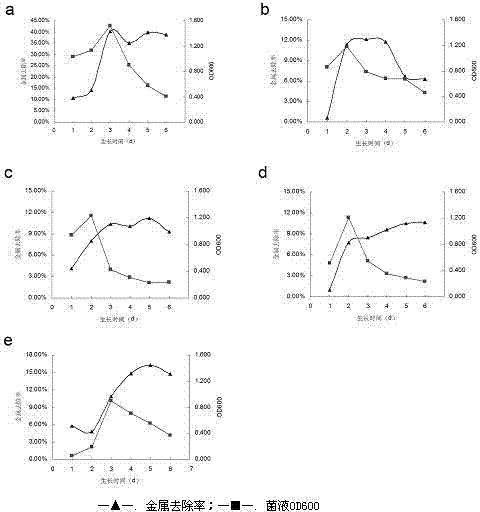
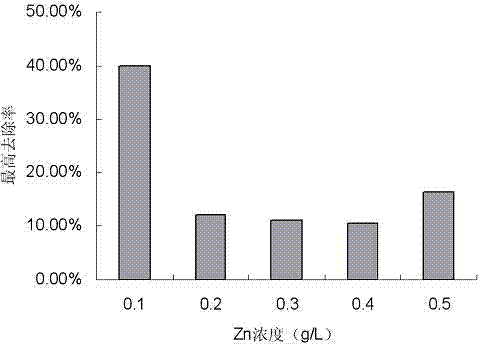
A strain of Pseudomonas aeruginosa and its application in removing heavy metal ions
InactiveCN103937702BEfficient removalNo secondary pollutionBacteriaWater contaminantsScreening methodPhysical chemistry
The present invention discloses a strain of Pseudomonas aeruginosa ZC6, which provides a heavy metal ion Zn<2+> removing effect. The method for screening Pseudomonas aeruginosa providing a heavy metal ion Zn<2+> removing effect from soil and water comprises: a, preparing a metal selective culture medium; b, carrying out enrichment acclimatization on a heavy metal resistance strain; c, screening and re-screening the heavy metal resistance strain; and d, detecting the heavy metal removing capability of the strain. According to the present invention, with the detection test on the heavy metal removing capability of the strain obtained by carrying out enrichment acclimatization on the heavy metal resistance strain with the metal ion-containing culture medium and purification, the reasonable and effective screening method for the heavy metal removing strain is established so as to obtain the strain suitable for requirements; and with application of the Pseudomonas aeruginosa ZC6 in the heavy metal pollution treatment, advantages of no secondary pollution, high treatment efficiency, wide application range, low cost and the like are provided.
Owner:曾泽华 +1
Type iii secretion system component protein pa1698 of pseudomonas aeruginosa
InactiveUS20100291070A1Practically preventPractically treatAntibacterial agentsAnimal cellsSecretionType three secretion system
An object is to provide an antibody and a vaccine composition which have an ability to practically prevent or treat a Pseudomonas aeruginosa infection, and which can cope with the diversity of clinical isolates derived from patients infected with Pseudomonas aeruginosa. According to the present invention, an antibody against a PA1698 protein that is a type III secretion system component protein of Pseudomonas aeruginosa or against a peptide of the protein, and a vaccine composition comprising the protein or the peptide are provided.
Owner:MEIJI SEIKA PHARMA CO LTD
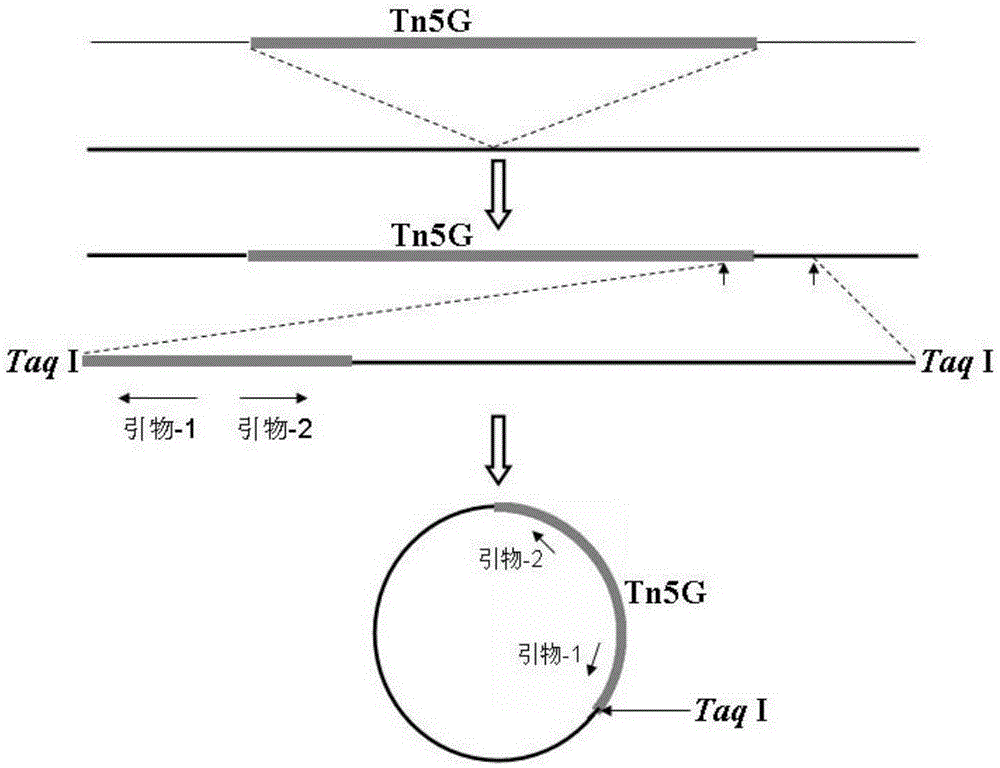
Genes of pseudomonas aeruginosa related to phage infection and application of genes
ActiveCN105400876AUnderstand molecular mechanismsTo achieve the effect of indirect treatment of viral infectionMicrobiological testing/measurementAnaplasma phagocytophilumAntiviral drug
The invention relates to functions of genes of pseudomonas aeruginosa and an application of the genes, wherein the gene BN889_05221, the gene PA0243, the gene PA3808, the gene PA1993 and the gene PA1115 of pseudomonas aeruginosa are the necessary host genes of phage infection, and are used for screening the relevant medicines for inhibiting the phage infection. According to the invention, through the study on the relevant host genes of phage infection, a molecular mechanism for the interaction between phage and host bacteria can be well understood, therefore, a theoretical basis is provided for the treatment on the phage, meanwhile, new target genes related to virus infection can be found, and assistance is provided for screening antiviral drugs.
Owner:TIANJIN UNIVERSITY OF SCIENCE AND TECHNOLOGY
Pseudomonas aeruginosa and pseudomonas aeruginosa-containing marine mammal vaccine
ActiveCN108251341AEffective homologous attack protectionImprove immunityAntibacterial agentsBacteriaActive componentMicrobiology
The invention discloses pseudomonas aeruginosa and a pseudomonas aeruginosa-containing marine mammal vaccine. A used strain is a pseudomonas aeruginosa DCP1 strain separated from a marine mammal, andhas a preservation number of CGMCC NO. 15418. The invention further discloses the marine mammal vaccine. An inactivated pseudomonas aeruginosa antigen of the marine mammal is used as an active component of the marine mammal vaccine. The invention further discloses a preparation method of the marine mammal vaccine. The preparation method comprises processes of separation of the strain, authentication, purification, antigen preparation, vaccine preparation and the like. The pseudomonas aeruginosa inactivated vaccine prepared through the preparation method disclosed by the invention is safe and reliable, can obviously increase the antibody level and has a wide application prospect.
Owner:BEIJING VBIOSCI INC +1
Genes related to Pseudomonas aeruginosa and phage infection and their application
ActiveCN105400876BAdsorption has no effectUnderstanding the Molecular Mechanisms of InteractionsMicrobiological testing/measurementAnaplasma phagocytophilumAntiviral drug
The invention relates to functions of genes of pseudomonas aeruginosa and an application of the genes, wherein the gene BN889_05221, the gene PA0243, the gene PA3808, the gene PA1993 and the gene PA1115 of pseudomonas aeruginosa are the necessary host genes of phage infection, and are used for screening the relevant medicines for inhibiting the phage infection. According to the invention, through the study on the relevant host genes of phage infection, a molecular mechanism for the interaction between phage and host bacteria can be well understood, therefore, a theoretical basis is provided for the treatment on the phage, meanwhile, new target genes related to virus infection can be found, and assistance is provided for screening antiviral drugs.
Owner:TIANJIN UNIV OF SCI & TECH
Primer, kit and method for detecting pseudomonas aeruginosa and plcH in water
InactiveCN107419013AReasonable compositionReasonable ratioMicrobiological testing/measurementDNA/RNA fragmentationBiologyGene
Owner:蔡先全
Pseudomonas aeruginosa and application of Pseudomonas aeruginosa in heavy metal ion removing
InactiveCN103937702AEfficient removalNo secondary pollutionBacteriaWater contaminantsScreening methodPhysical chemistry
The present invention discloses a strain of Pseudomonas aeruginosa ZC6, which provides a heavy metal ion Zn<2+> removing effect. The method for screening Pseudomonas aeruginosa providing a heavy metal ion Zn<2+> removing effect from soil and water comprises: a, preparing a metal selective culture medium; b, carrying out enrichment acclimatization on a heavy metal resistance strain; c, screening and re-screening the heavy metal resistance strain; and d, detecting the heavy metal removing capability of the strain. According to the present invention, with the detection test on the heavy metal removing capability of the strain obtained by carrying out enrichment acclimatization on the heavy metal resistance strain with the metal ion-containing culture medium and purification, the reasonable and effective screening method for the heavy metal removing strain is established so as to obtain the strain suitable for requirements; and with application of the Pseudomonas aeruginosa ZC6 in the heavy metal pollution treatment, advantages of no secondary pollution, high treatment efficiency, wide application range, low cost and the like are provided.
Owner:曾泽华 +1
Method for killing pseudomonas aeruginosa
InactiveCN101537197BQuick killEfficient killingLavatory sanitoryDisinfectionLow temperature plasmaToxicology
The invention relates to the field of disinfection and sterilization, which discloses a method for killing pseudomonas aeruginosa. The method is achieved only by putting a carrier infected with the pseudomonas aeruginosa in plasma environment at a low temperature of between 10 DEG C and 50 DEG C for killing the pseudomonas aeruginosa for 30-100 seconds, wherein the low-temperature plasma environment is generated by a radio frequency plasma discharge device.
Owner:XI AN JIAOTONG UNIV
A kind of Pseudomonas aeruginosa bacterial strain and application thereof
InactiveCN105754908BIncrease vitalityThe cultivation method is simpleBacteriaMicroorganism based processesNicotiana tabacumPesticide residue
Owner:普洱学院 +1
A kind of Pseudomonas aeruginosa and application thereof
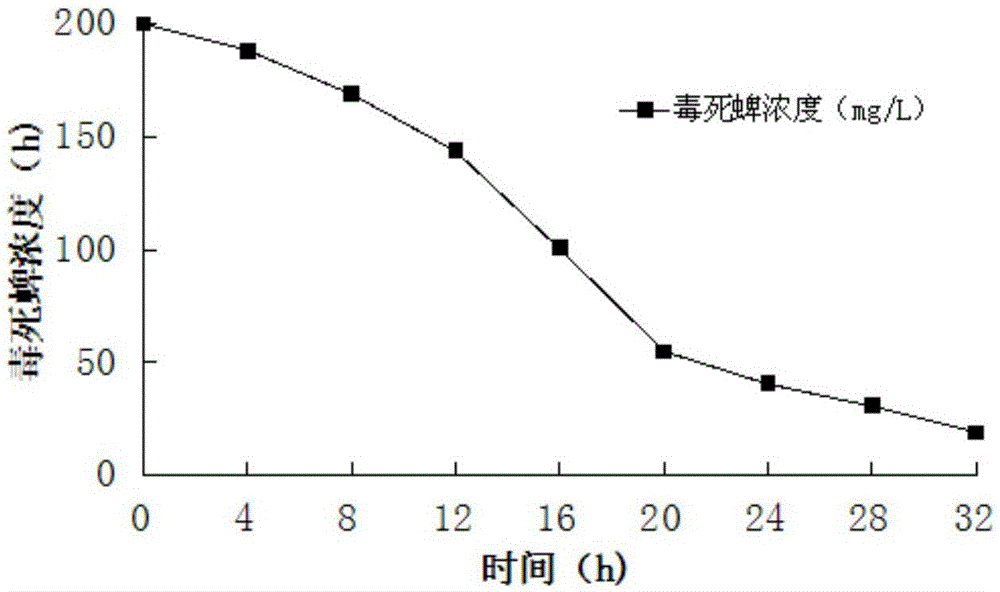
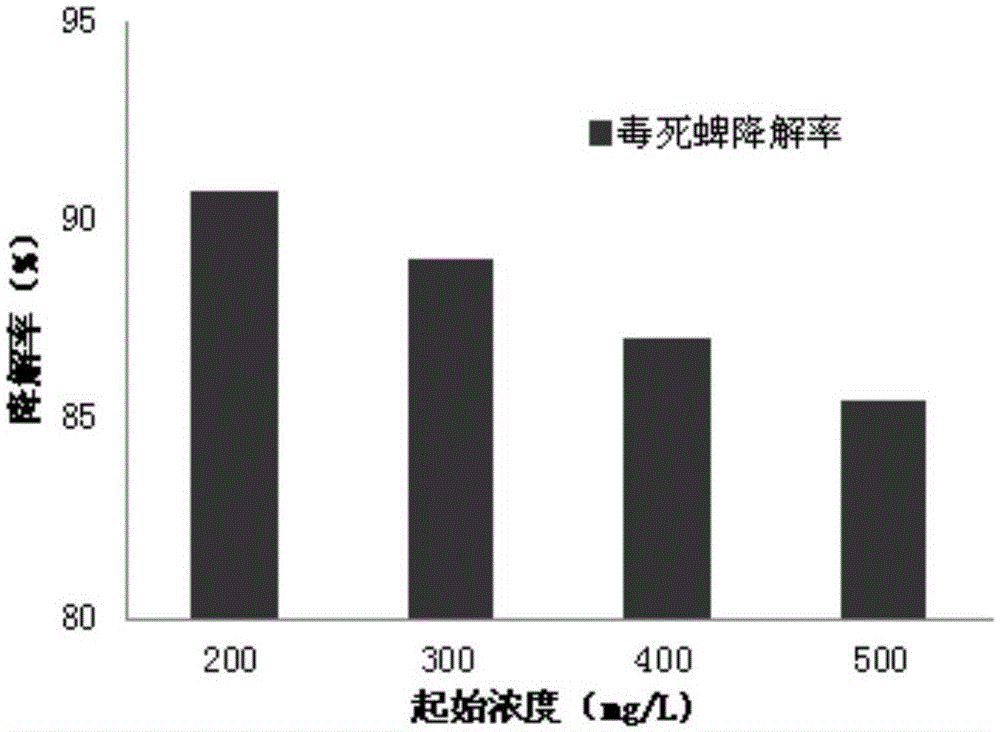
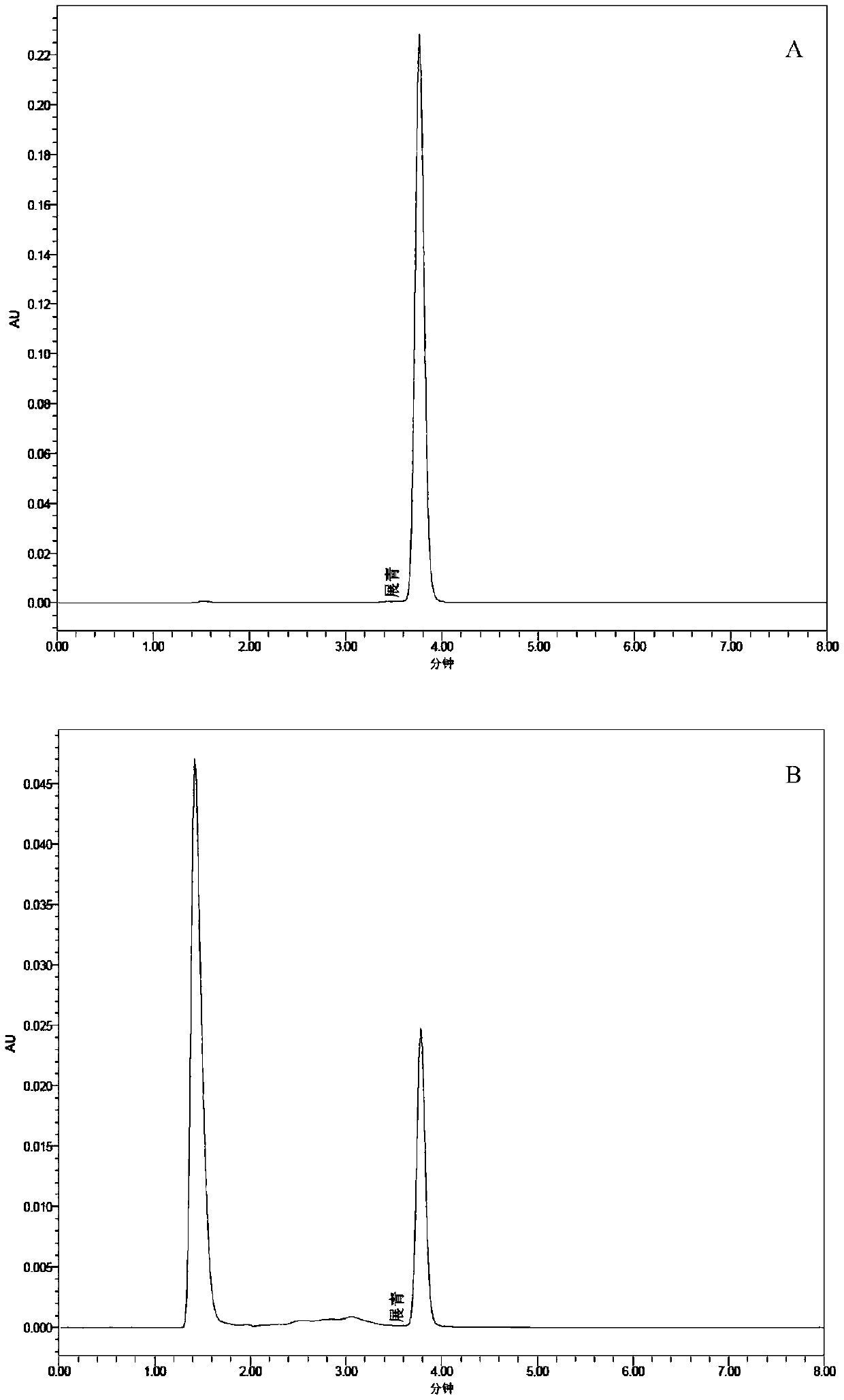
ActiveCN103820370BReduce manufacturing costGood removal effectBacteriaWater contaminantsMicroorganismChlorpyrifos
The invention discloses a pseudomanas aeruginosa. The pseudomonas aeruginosa is named as pseudomonas aeruginosa DSP-05 preserved in the general microorganism center of China Committee for Culture Collection of Microorganisms with the preservation number of CGMCC No.8390 and the preservation date of October 24, 2013. According to the invention, the pseudomanas aeruginosa is mainly applied to degradation of chlorpyrifos in wastewater, and suitable for popularization and utilization in treating the wastewater that contains chlorpyrifos; immobilized glomerular chlorpyrifos prepared by adopting bacterial strains has the advantages of better effect, low production cost, convenience for utilization and good removal effect; the pseudomonas aeruginosa plays an important role in protecting ecological environment and human health, and reducing the wastewater treatment cost; the pseudomonas aeruginosa DSP-05 is simple in culture condition and easy to keep and for industrial production, and has a good development and application prospect.
Owner:JIANGSU POLYTECHNIC COLLEGE OF AGRI & FORESTRY
Application of Pseudomonas aeruginosa in degrading patulin
The invention relates to the field of microorganisms, and specifically discloses a new application of pseudomonas aeruginosa, namely the new application of the pseudomonas aeruginosa to patulin degradation. According to the invention, it is proved by experiments that the pseudomonas aeruginosa can degrade the patulin effectively. The pseudomonas aeruginosa has a good application prospect in developing both of new biodegradation microbial preparations and new biodegradation sterile preparations.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Preparation of pseudomonas aeruginosa fermentation extract and application thereof in printing and dyeing fixing agent
InactiveCN109680526AHigh yieldHigh activityMicroorganismsMicroorganism based processesWater bathsAdditive ingredient
The invention discloses a method for preparing a pseudomonas aeruginosa fermentation extract and application thereof in a printing and dyeing fixing agent. The preparation method comprises the following steps: performing constant-temperature water bath treatment on a pseudomonas aeruginosa fermentation solution, cooling, regulating to acidity, and stirring; filtering with plate-frame, performing flash drying on filtrate to obtain a solid fermentation solution; extracting the solid fermentation solution with isopropyl alcohol; transferring the extract into a separating funnel, adding ethyl acetate into the funnel, sufficiently oscillating, and standing; performing rotary evaporation and concentration on supernate, and performing two-stage countercurrent extraction and spray drying on the rotary evaporated concentrate with a centrifuge to obtain the pseudomonas aeruginosa fermentation extract. The pseudomonas aeruginosa fermentation extract can be used for effectively improving the colorfastness index of textiles by applying to a printing and dyeing fixing agent, and does not contain formaldehyde and other harmful ingredients to improve the use performance of the printing and dyeingfixing agent.
Owner:黄敏
A kind of Pseudomonas aeruginosa and application thereof
ActiveCN104371940BStrong oil displacement performanceReduce interfacial tensionBacteriaMicroorganism based processesMicrobial oilMetabolite
The present invention relates to a kind of Pseudomonas aeruginosa and its application; The Pseudomonas aeruginosa QHH S1-27-2, its microbial preservation number is CCTCC NO: M2012468; it is applied to high salinity oil reservoir microbial oil recovery Middle; Pseudomonas aeruginosa of the present invention can grow with crude oil as the only carbon source, and the biosurfactant component in its metabolites can reduce the oil-water interfacial tension, increase the effective permeability of the reservoir to a certain extent, and improve the injection water Oil displacement efficiency, enhanced oil recovery.
Owner:PETROCHINA CO LTD
A method for the inactivation of Pseudomonas aeruginosa and Sphingomonas paucimobilis based on the growth loop of water supply network
ActiveCN104944538BWater/sewage treatment using germicide/oligodynamic-processWater/sewage treatment by oxidationUrban water supplySphingomonas paucimobilis
A method for the inactivation of Pseudomonas aeruginosa and Sphingomonas paucimobilis based on the growth ring of water supply pipe network, which involves two pathogenic bacteria Pseudomonas aeruginosa and Sphingomonas paucimobilis in drinking water Bacteria inactivation method. It aims to solve the problem of poor inactivation effect of Pseudomonas aeruginosa and Sphingomonas paucimobilis in existing drinking water. Method: Add α‑FeOOH and H2O2 to the clean water tank of the city water supply plant, and the process is completed. The cost of H2O2 in the present invention is low, and the α-FeOOH material is cheap and easy to obtain, and it is the main component of the growth ring. After one addition, it can continue to exist in a large amount in the urban water supply network. In the later stage, only H2O2 needs to be added. The requirements are simple, the energy loss is low, and the input and operating costs are saved. The Fentonoid composed of α‑FeOOH and H2O2 has obvious inactivation effect on Pseudomonas aeruginosa and Sphingomonas paucimobilis present in the filtered water.
Owner:HARBIN INST OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com