Pseudomonas aeruginosa gene and DNA vaccine thereof
A Pseudomonas aeruginosa, DNA vaccine technology, applied in the field of molecular biology and infection immunity, can solve the problem of low immune efficacy of DNA vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
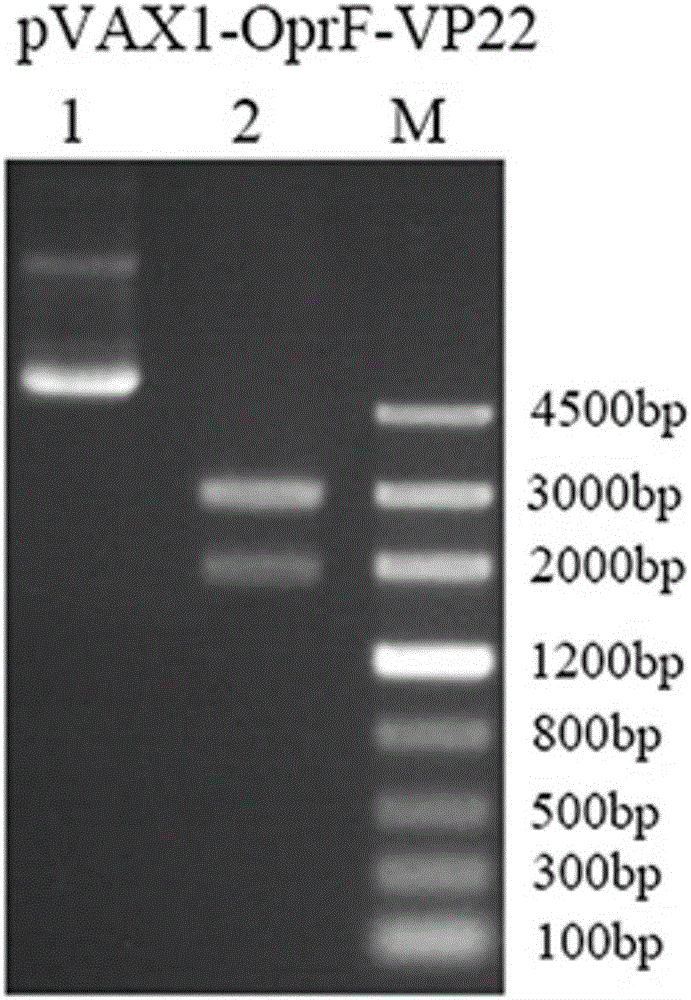
[0021] Embodiment 1 pVAX1-OprF-VP22 construction method
[0022] Construction of Pseudomonas aeruginosa DNA vaccine pVAX1-OprF-VP22 by overlapping PCR
[0023] 1. PCR amplification of the OprF gene: According to the instructions of the bacterial genome extraction kit of Beijing Tiangen Biological Co., Ltd., the Pseudomonas aeruginosa genome was extracted from Pseudomonas aeruginosa PAO1, and then the open reading frame 1~ of the OprF gene sequence was amplified by primers. 1050bp (GenBank: NC_002516.2). P3: 5′-CGC GGATCC ACCATGAAACTGAAGAACAC-3'), where the BamHI restriction site is underlined; P7: 5'-CTGAAGCCAAGATGACCTCTCGCCG-3'. PCR parameters: pre-denaturation temperature and time 94°C 3min; denaturation temperature and time 94°C 30sec, annealing (refolding) temperature and time 55°C 30sec, extension temperature and time 72°C 1min, a total of 30 cycles; stop after 72°C 5min reaction.
[0024] 2. PCR amplification of the VP22 gene: pcDNA3-VP22 was used as a template, and...
Embodiment 2
[0026] Example 2 Immunization of mice with pVAX1-OprF-VP22 DNA vaccine and preparation of mouse antiserum
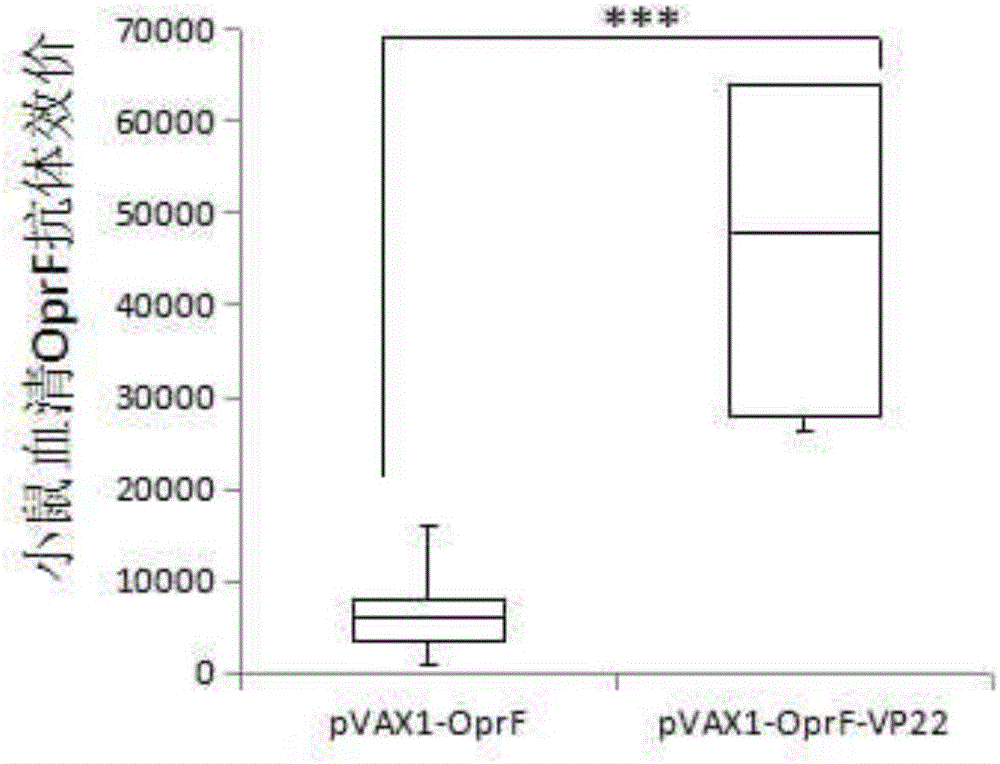
[0027] 1. Mice immunized with DNA vaccine: SPF level 6-8 week old healthy female BALB / c mice were randomly divided into PBS immunization group (negative control), pVAX1-OprF, pVAX1-OprF-VP22 groups, 8 mice in each group . The mice in the pVAX1-OprF and pVAX1-OprF-VP22 groups were injected intramuscularly with 20ug of the corresponding plasmid, and the negative control group was injected with PBS. The mice were immunized three times with an interval of 2 weeks.
[0028] 2. Preparation of mouse serum: The mice were killed 1 week after the last immunization, and the venous blood was collected by enucleation. The collected blood samples were placed at 37°C for 1 hour and then fully coagulated overnight at 4°C. Centrifuged at 4,000rpm for 10 minutes at 4°C to collect serum , Store at -70°C after aliquoting.
[0029] 3. Preparation of mouse T lymphocytes: the mice were killed ...
Embodiment 3
[0030] Example 3 pVAX1-OprF-VP22 enhances the humoral immunity of OprFDNA vaccine
[0031] 1. ELISA indirect method determination:
[0032] 1) Antigen coating: Dilute the purified OprF with 0.05M pH9.6 carbonate buffer at an optimal dilution (1ug / ml), add it to a polystyrene microplastic plate, 100ul per well, 4 ℃ coated overnight (16h-24h).
[0033] 2) Washing: Shake off the liquid in it, pat dry on absorbent paper, add washing solution, 300ul / well, shake, place at room temperature for 5min, and wash 3 times in total.
[0034] 3) Blocking: blocking solution 300ul / well. Incubate at 37°C for 2 hours, wash 3 times after blocking (same as before).
[0035] 4) Primary antibody incubation: Add serially diluted serum to be tested (starting at 1:500), add 100ul to each well (double wells), set blank control wells and negative controls at the same time, and incubate at 37°C for 1h.
[0036] 5) Wash 3 times (same as before).
[0037] 6) Secondary antibody incubation: dilute the se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com