APMV (avian paramyxovirus) fusion protein, preparation method and application thereof and APMV vaccine for pigeons
A technology of avian paramyxovirus and fusion protein, applied in the direction of antiviral immunoglobulin, veterinary vaccine, biochemical equipment and methods, etc., can solve the disadvantages of emergency vaccination and reduce the cost of vaccines, have potential infection risks, and are difficult to promote Application and other issues to achieve the effect of preventing the prevalence and spread of infection, good antigenicity, and reducing morbidity and mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The present invention also provides a kind of above-mentioned avian paramyxovirus fusion protein, the preparation method of above-mentioned avian paramyxovirus fusion protein or the application of the avian paramyxovirus fusion protein prepared by the preparation method, including the following (x1)-(x6 ) in at least one: (x1) preparation of pigeon subunit dual vaccine; (x2) preparation of vaccine for poultry viral diseases; (x3) preparation of antibody to avian paramyxovirus; (x4) preparation of antigen of avian paramyxovirus (x5) preparing reagents and / or kits for detecting avian paramyxovirus; (x6) preparing reagents and / or kits for detecting avian paramyxovirus antibodies.
[0052] The avian paramyxovirus fusion protein provided by the present invention expresses the HN gene of pigeon type 1 paramyxovirus, that is, Newcastle disease virus and avian paramyxovirus type 7 in series, and the avian paramyxovirus fusion protein not only contains pigeon type 1 paramyxovirus...
Embodiment 1
[0058] Embodiment 1 Expression of avian paramyxovirus fusion protein
[0059] 1.1 Synthesis and amplification of the target gene
[0060] Obtain the whole viral RNA sequence of NDV and APMV-7 through GenBank, and find the DNA sequence encoding the HA gene, wherein the amino acid sequence of the HN gene encoding NDV is shown in SEQ ID NO.1, and the nucleotide sequence is shown in SEQ ID NO.4 shown; the amino acid sequence of the HN gene encoding AMPV-7 is shown in SEQ ID NO.2, and the nucleotide sequence is shown in SEQ ID NO.5.
[0061] After codon optimization of the gene sequence according to the expression host, a flexible fusion protein linking peptide (PPSPS) was selected 2 The HN gene of NDV and the HN gene of APMV-7 were connected, and the sequence was named NDAP-HN, and its amino acid sequence was shown in SEQ ID NO.3, and was sent to a biological company for synthesis.
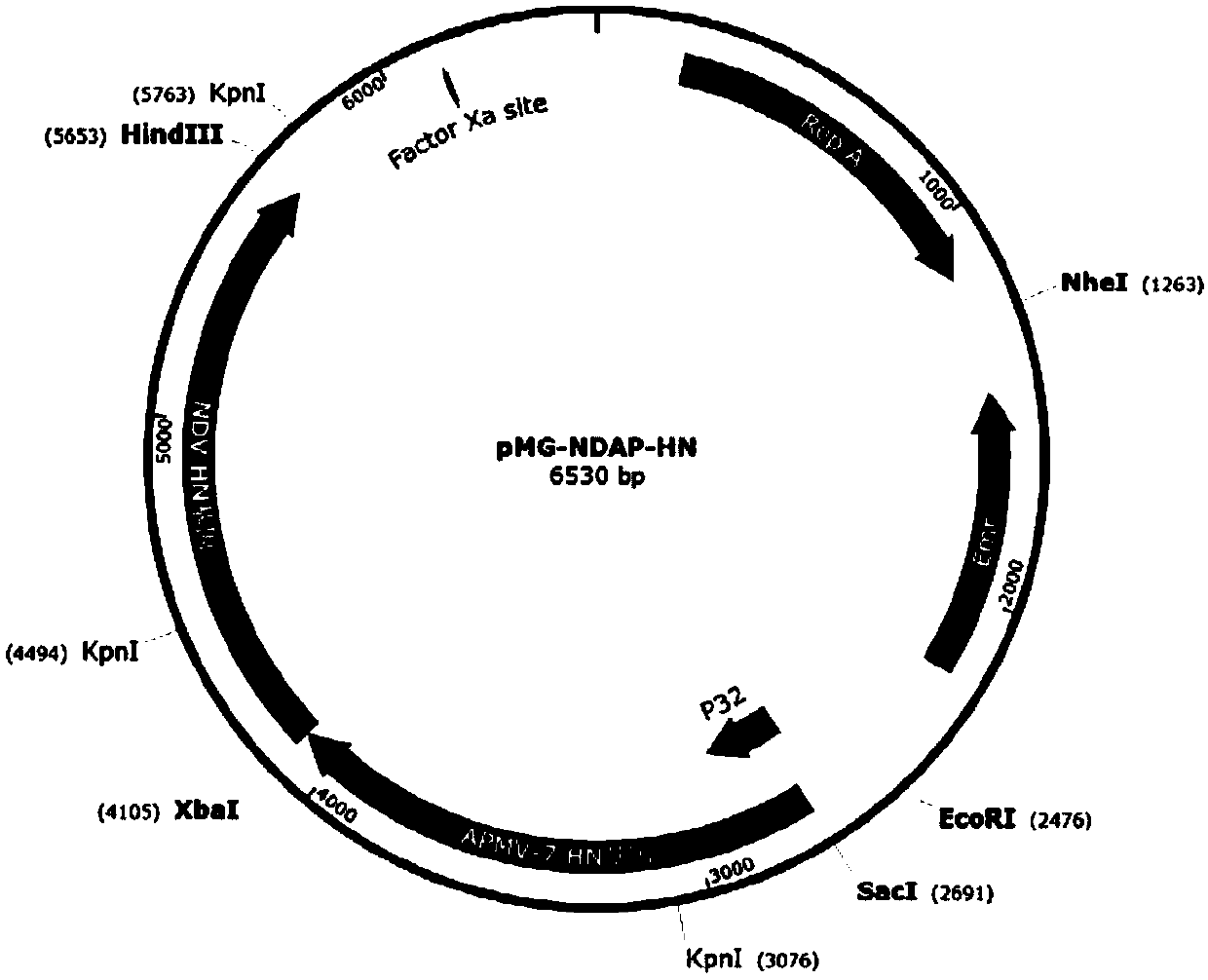
[0062] 1.2 Expression vector construction
[0063] The synthetic NDAP-HN gene and pMG36e expres...
Embodiment 2
[0076] The preparation of embodiment 2 vaccine
[0077] Clean the vials and wrap them up, sterilize at 121°C for 15 minutes, and dry them for later use. Multi-valve vial stoppers ready to be sterilized. Inoculate the activated single colony into the test tube containing the culture medium, and after cultivation, add the freeze-dried protective agent (20g milk powder + 80g pure water) and mix evenly at a ratio of 1:3; add 2ml of the mixed solution to each bottle and cover it Cork (from the gap between the cork flaps), with the strain number written on the bottle. Store the aliquoted mixture in a -20°C refrigerator overnight. The next day, the mixed solution was frozen in a -80°C refrigerator for 2 h. Then put the vials containing the mixed solution together into a freeze dryer for extraction and pre-freeze drying. Freeze-dry according to the operating procedures of the freeze-dryer. Finally, the vial was taken out, capped, and the effect of freeze-drying was observed, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com