Pseudomonas aeruginosa of minks and application of pseudomonas aeruginosa
A technology of mink Pseudomonas aeruginosa and Pseudomonas aeruginosa, applied in the direction of bacteria, antibacterial drugs, veterinary vaccines, etc., can solve the problems of weak immunogenicity and low immune cross-protection, and reduce morbidity and mortality , good immunogenicity, strong immunity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1.1 Isolation and purification method of Pseudomonas aeruginosa bacterial strain
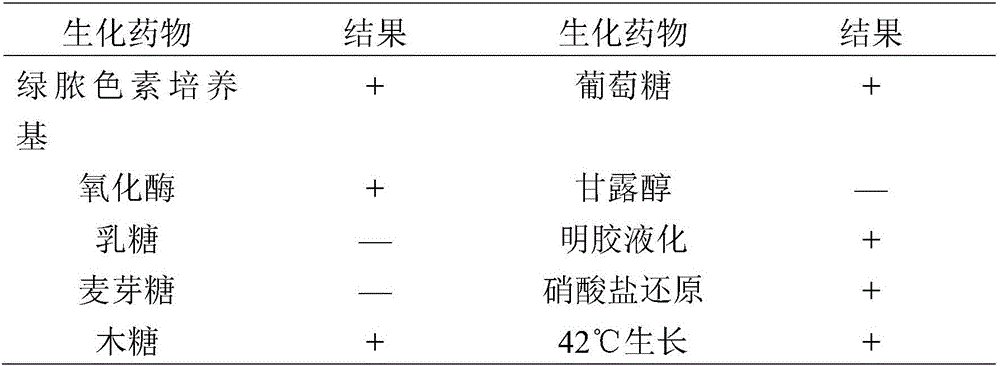
[0019] The affected minks were selected from a large mink farm suspected of hemorrhagic pneumonia in Liaoning Province, and the lungs, liver and heart blood of the sick minks were aseptically collected and inoculated on cetyltrimethylammonium bromide agar medium (NAC) , placed in a 37°C incubator for 16h to 18h, picked round, smooth, moist, and flat colonies for Gram staining and microscopic examination, and selected Gram-negative small bacilli for streaking and pure culture.
[0020] Stratify the pure culture strains on fresh blood agar plate and NAC plate respectively, culture at 37°C for 16h~18h, choose to produce green fluorescent pigment on hexadecanetrimethylammonium bromide medium, and produce β on blood agar medium Hemolyzed small colonies were purified and cultured.
[0021] According to the biochemical identification test requirements of Pseudomonas aeruginosa, the two strains ...
Embodiment 2
[0047] Application of mink Pseudomonas aeruginosa type B LN03 strain in preparation of inactivated vaccine.
[0048] The preservation number of the strain is taken as the Pseudomonas aeruginosa type B LN03 strain with the preservation number CCTCCNO: M2016068. After activation, the culture medium is inoculated respectively, the bacterial liquid is collected after cultivation, inactivated by formaldehyde solution, and mixed with propolis adjuvant to prepare inactivated Propolis vaccine.
[0049] The preferred operation steps are as follows:
[0050] 2.1 Strain selection
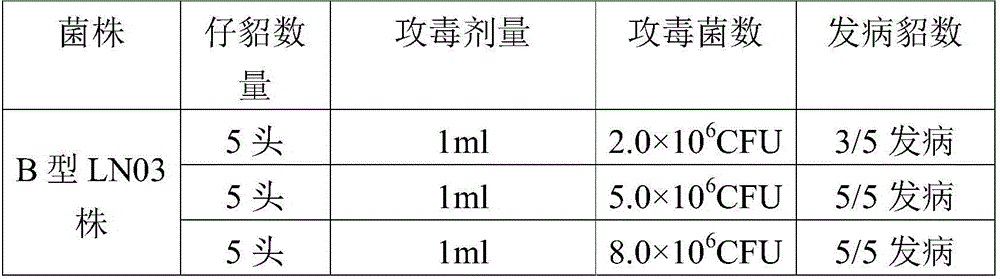
[0051] The strain used for seedling production was Pseudomonas aeruginosa type B LN03 strain, which was stable in colony morphology, bacterial characteristics, biochemical characteristics, and culture characteristics, and had strong pathogenicity to minks. 1.0mL bacterial liquid (5.0×10 6 CFU / mL) can cause 100% of 21-42 day-old mink disease. The immunogenicity of mink Pseudomonas aeruginosa type B LN03 stra...
Embodiment 3
[0092] Application of mink Pseudomonas aeruginosa type B LN03 strain in the preparation of inactivated vaccine.
[0093] The preservation number of the strain is taken as Pseudomonas aeruginosa type B LN03 strain with the preservation number CCTCCNO: M2016068. After activation, the culture medium is inoculated respectively, the bacterial liquid is collected after cultivation, inactivated by formaldehyde solution, and mixed with water adjuvant to prepare inactivated vaccine.
[0094] The preferred operation steps are as follows:
[0095] 2.1 Strain selection
[0096] The strain used for seedling production was Pseudomonas aeruginosa type B LN03 strain, which was stable in colony morphology, bacterial characteristics, biochemical characteristics, and culture characteristics, and had strong pathogenicity to minks. 1.0mL bacterial liquid (5.0×10 6 CFU / mL) can cause 100% of 21-42 day-old mink disease. The immunogenicity of mink Pseudomonas aeruginosa type B LN03 strain was good,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com