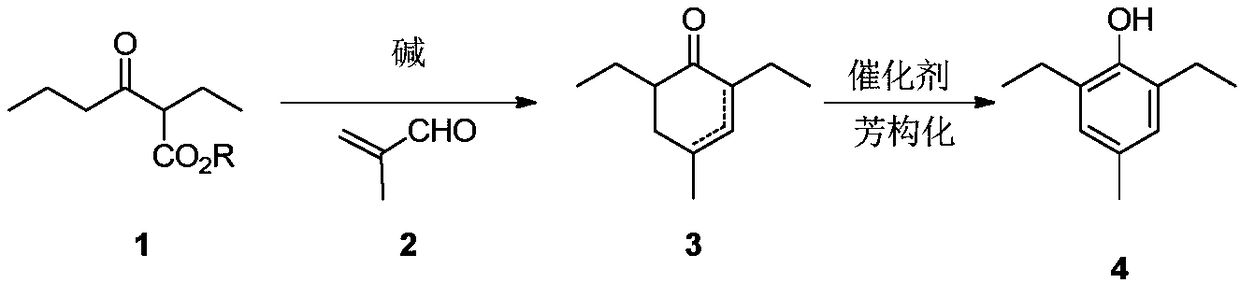
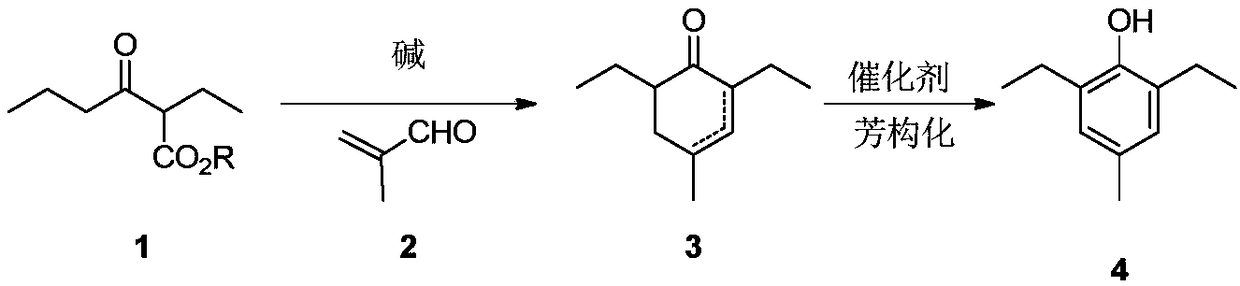
Preparation method of 2,6-diethyl-4-methylphenol
A technology of methyl phenol and diethyl, which is applied in the field of preparation of 2,6-diethyl-4-methylphenol, can solve the problems of excessive three wastes, low yield, long route, etc., and achieve safe operation and high raw material The effect of easy availability and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
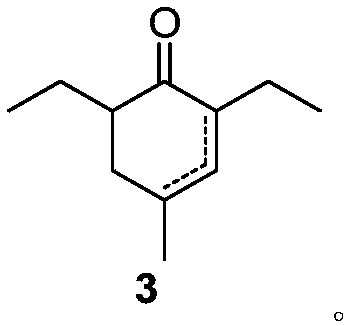
[0020] Example 1: Preparation of 2,6-diethyl-4-methyl-2-cyclohexenone
[0021] Add 6.2g (0.115mol) of sodium methoxide into methanol, stir until the sodium methoxide dissolves, add 7.6g (0.048mol) of the raw material 2-ethyl-3-oxohexanoic acid methyl ester, raise the temperature to 60°C for reaction, and dropwise add formaldehyde 5.0 g (0.072 mol) of acrolein in methanol. After the reaction was complete, the temperature was lowered, ethyl acetate was added, washed with water, concentrated and purified to obtain 5.8 g of the product 2,6-diethyl-4-methyl-2-cyclohexenone with a yield of 72%. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.44-6.42(m,1H,),2.58-2.53(m,1H,),2.22-2.12(m,2H),2.10-2.05(m,1H),1.14-1.12( m,3H), 1.01-0.91(m,10H). 13 C NMR (CDCl 3 ,125MHz): δ201.46, 148.82, 139.94, 47.81, 35.86, 31.83, 22.386, 22.08, 21.55, 13.51, 12.79.
Embodiment 2
[0022] Example 2: Preparation of 2,6-diethyl-4-methyl-2-cyclohexenone and 2,6-diethyl-4-methyl-3-cyclohexenone
[0023] Add 4.6g (0.115mol) of sodium hydroxide into tetrahydrofuran, add 8.3g (0.048mol) of ethyl 2-ethyl-3-oxohexanoate as raw material, raise the temperature to reflux, and add 5.0g (0.072mol) of methacrolein dropwise. mol) solution in tetrahydrofuran. After the reaction is complete, lower the temperature, add ethyl acetate, wash with water, and concentrate to obtain 6.5g of 2,6-diethyl-4-methyl-2-cyclohexenone and 2,6-diethyl-4-methyl- The mixture of 3-cyclohexenone, the yield is 81%, and the ratio of the two as determined by GC-MS is 90:10.
Embodiment 3
[0024] Example 3: Preparation of 2,6-diethyl-4-methyl-2-cyclohexenone
[0025] Add 40.0g (1.00mol) of sodium hydroxide into THF / H 2 In the mixed solution of O, stir until the sodium hydroxide is dissolved, add 93.1 g (0.50 mol) of ethyl 2-ethyl-3-oxohexanoate as raw material, raise the temperature to reflux reaction, add dropwise 42.0 g (0.60 mol) of methacrolein mol) THF solution. After the reaction was complete, the temperature was lowered, ethyl acetate was added, washed with water, concentrated and purified to obtain 58.2 g of the product 2,6-diethyl-4-methyl-2-cyclohexenone with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com