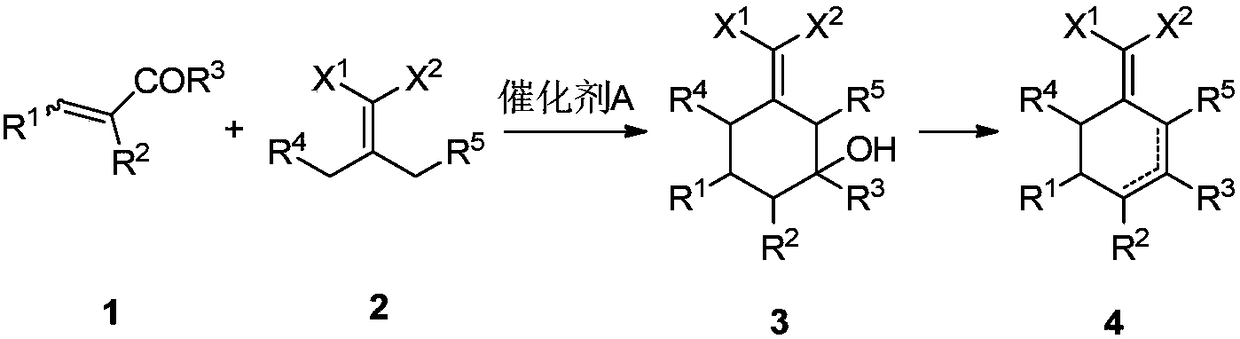
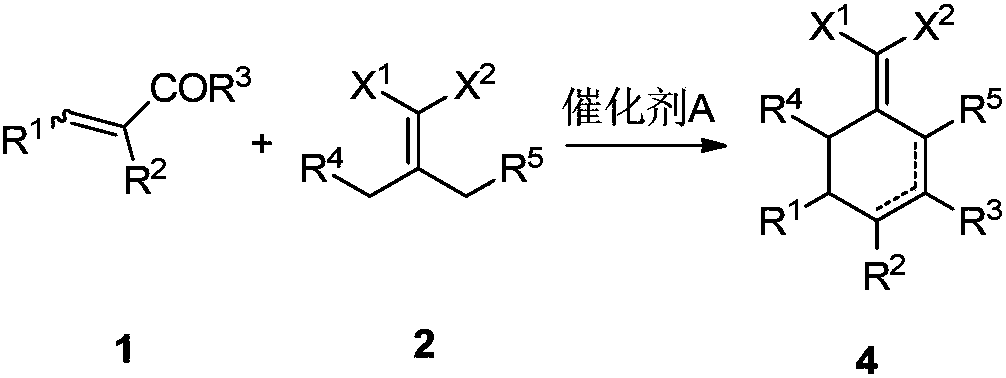
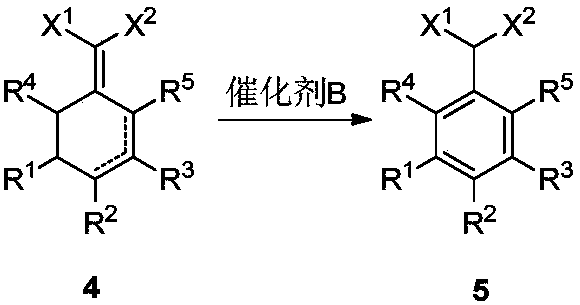
Preparation method for 2-(cyclohexenyl) malonic acid derivative and application thereof
A technology of cyclohexenyl and malonic acid, which is applied in the direction of botanical equipment and methods, applications, and preparation of carboxylic acid nitriles, can solve the problems of unsuitability for industrial production, limited application, high cost, etc., and is beneficial to industrial production, The effect of less waste and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] Preparation of 2-(4-heptylene)malononitrile
[0032] Add 65.0 g (0.569 mol) of 4-heptanone, 39.4 g (0.569 mol) of malononitrile, 6.6 g (0.086 mol) of ammonium acetate, 10.3 g (0.171 mol) of acetic acid, and toluene into the reaction flask in sequence, and reflux for water separation reaction After the reaction was complete, the temperature was lowered, washed with water, concentrated and purified to obtain 84.9 g of the product 2-(4-heptylene)malononitrile, with a yield of 92%. 1 H NMR (CDCl 3 , 500MHz, TMS): δ2.57-2.53 (m, 4H), 1.64-1.60 (m, 4H), 1.02 (t, J=7.5Hz, 6H). 13 C NMR (CDCl 3 ,125MHz): δ186.08, 111.88, 85.91, 37.47, 21.44, 12.81.
[0033] Preparation of 2-(1-(4-methoxyphenyl)-2-propylene)malononitrile
[0034] 82.1g (0.50mol) of 1-(4-methoxyphenyl)-2-propanone, 33.0g (0.50mol) of malononitrile, 5.8g (0.075mol) of ammonium acetate, 9.0g (0.15mol) of acetic acid, Add toluene into the reaction flask in turn, reflux and divide water for reaction. After the rea...
Embodiment 1
[0035] Example 1: Preparation of 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene)malononitrile
[0036] Add 24.3g (0.15mol) of 2-(4-heptylene)malononitrile, 10.5g (0.15mol) of 2-methacrolein and 15.2g (0.15mol) of triethylamine into toluene in turn, and heat up to reflux for reaction until the reaction is complete. Cool down, wash with 1N dilute hydrochloric acid, dry, concentrate, and purify to obtain 25.7 g of the product 2-(2,6-diethyl-4-methyl-2-en-1-cyclohexylidene) malononitrile with a yield of 80 %. 1 H NMR (CDCl 3 ,500MHz,TMS):δ6.14-6.14(m,1H),3.08-3.04(m,1H),2.82-2.75(m,1H),2.57-2.46(m,2H),2.04-2.01(m, 1H), 1.56-1.51(m, 2H), 1.48-1.41(m, 1H), 1.12-1.01(m, 6H), 1.00-0.98(m, 3H). 13 C NMR (CDCl 3 ,125MHz): δ175.12, 148.74, 134.78, 113.99, 113.74, 43.75, 34.75, 28.13, 16.55, 15.52, 20.91, 13.59, 11.98.
Embodiment 2
[0037] Example 2: 2-(2,6-diethyl-4-methyl-2-ene-1-cyclohexylene)malononitrile and 2-(2,6-diethyl-4-methyl- Preparation of 3-ene-1-cyclohexylene)malononitrile
[0038] Add 25.0 g (0.154 mol) of 2-(4-heptylethylene) malononitrile, 14.0 g (0.200 mol) of 2-methacrolein and 15.6 g (0.154 mol) of triethylamine into toluene in sequence, and heat up to reflux for reaction until the reaction is complete. Cool down, wash with 1N dilute hydrochloric acid, dry, and concentrate the solvent to obtain 2-(2,6-diethyl-4-methyl-2-en-1-cyclohexylene)malononitrile and 2-(2,6-di 30.4 g of a mixture of ethyl-4-methyl-3-en-1-cyclohexylene)malononitrile. The ratio of the two was 91:9 by GC-MS analysis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com