Pharmaceutical composition for treating acute kidney injury
A pharmaceutical composition, kidney injury technology, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve the problems of ineffective treatment of stem cells, consumption of medical resources, inability to treat kidney damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0056] "Preparation Example 1" (Preparation of platelet-rich fibrin release solution)
[0057] First, 10-week-old New Zealand white rabbits (New Zealand White Rabbit, purchased from Weixinhang) were treated with 50 mg / mL of Zoletil ( 50, Virbac, France) and Xylazine at a concentration of 20mg / mL ( Gongyuan Pharmaceuticals, Taiwan) mixed with a 1:1 ratio, intramuscularly injected with a dose of 0.25mL / kg for general anesthesia, so that the white rabbits were sedated and reached a state of shallow anesthesia. Next, the hair near the neck of the rabbit was shaved clean, and the skin of the neck of the rabbit was wiped from the inside to the outside with cotton dipped in a mixture of 10% iodine and 75% ethanol for disinfection.
[0058] After the disinfection is completed, use a 10mL syringe with an 18G needle to collect fresh blood from the external jugular vein in the neck of the rabbit without adding an anticoagulant.
[0059]Immediately transfer the extracted fresh rabbit ...
preparation example 2
[0063] "Preparation Example 2" (Preparation of Adipose Stem Cells)
[0064] First, 8-week-old healthy SD strain rats without any obvious trauma were treated with Zoletil at a concentration of 50 mg / mL ( 50, Virbac, France) and Xylazine at a concentration of 20mg / mL ( Gongyuan Pharmaceuticals, Taiwan) mixed solution in a ratio of 1:1 was injected intramuscularly at a dose of 0.25mL / kg, and the rats were generally anesthetized. Then, the hair at the hind limbs of the rats was shaved, and the skin of the hind limbs of the rats was wiped from the inside to the outside with cotton dipped in a mixture of 10% iodine and 75% ethanol to completely sterilize.
[0065] The sterilized rats were subjected to liposuction under a sterile environment. The adipose tissue extracted from the abdominal cavity of the rat was washed three times with PBS, then added with type I collagenase, and shaken at room temperature for one hour. Centrifuge at 3000 rpm for 10 minutes.
[0066] After centr...
Embodiment 1
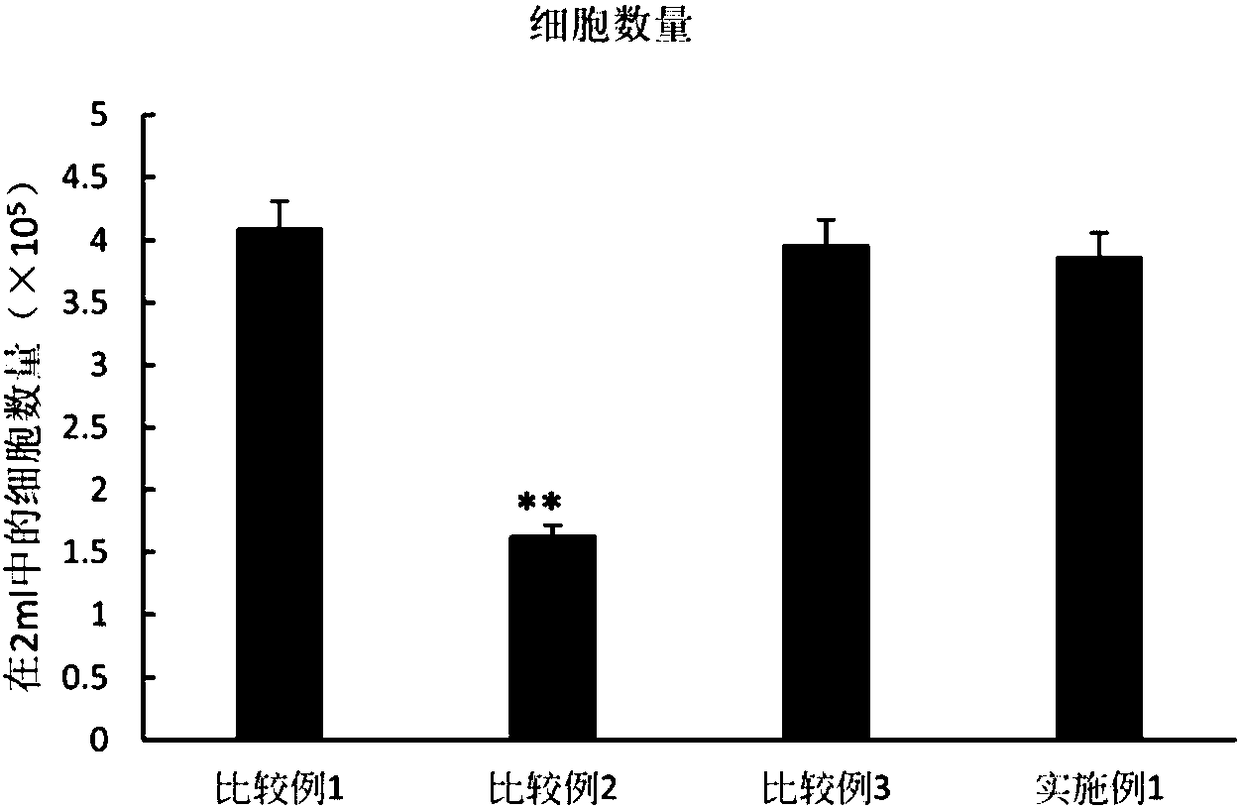
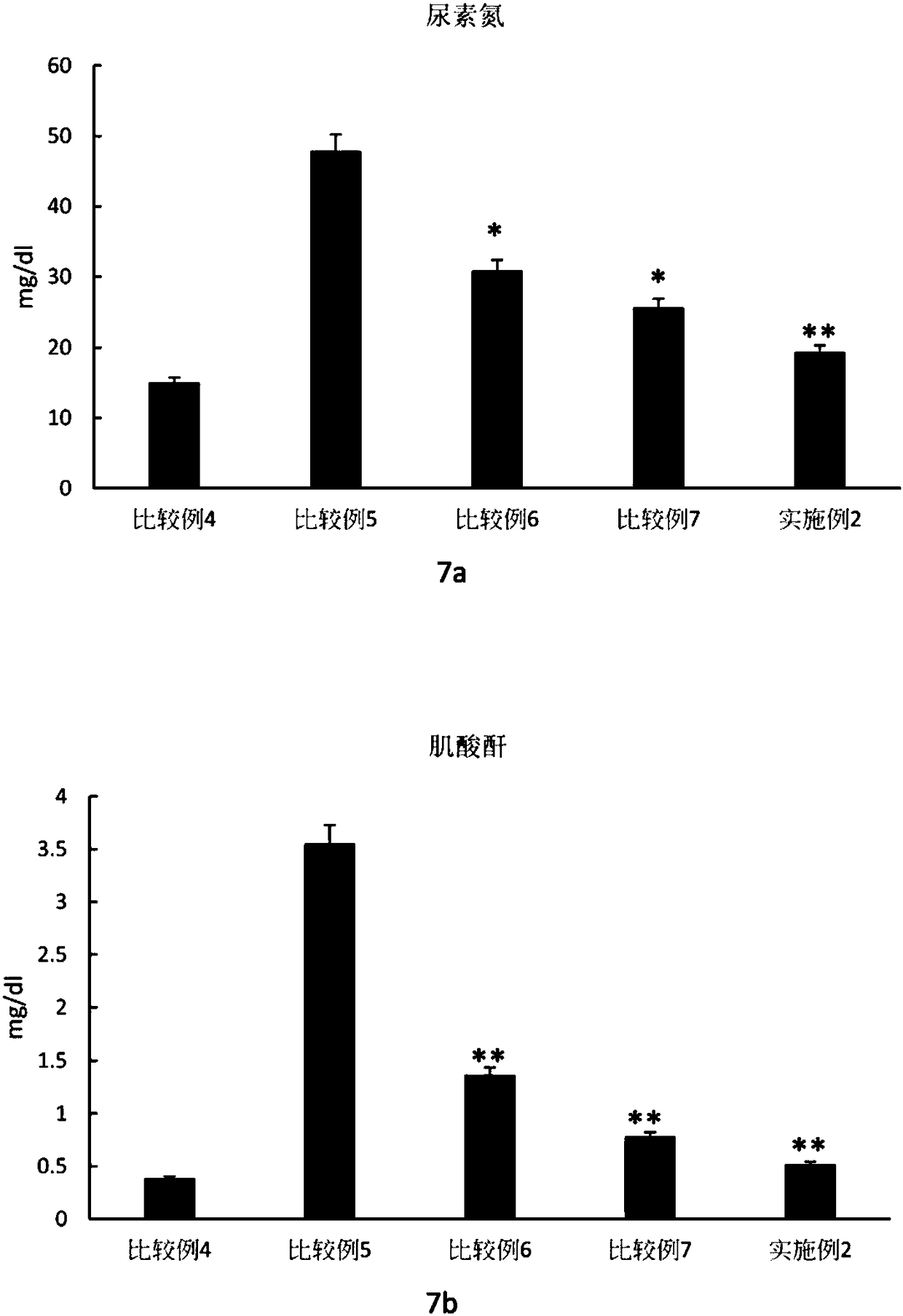
[0086] "Example 1" (kidney tissue+PRFr+adipose stem cell R of acute kidney injury)
[0087] First, acute kidney injury kidney tissue homogenate (AKI-KHS) was prepared as an experimental material. The rats after renal ischemia reperfusion (Ischemia Reperfusion, IR) operation were executed and their kidneys were removed. Acute kidney injury tissue homogenate (AKI-KHS), and then the acute kidney injury tissue homogenate (AKI-KHS) was centrifuged at a constant temperature of 4° C. at a speed of 20,000 rpm for 15 minutes. Afterwards, the supernatant was taken out and filtered through a sieve with a pore size of 30 μm to obtain an AKI-HHS filtrate. Finally, put the filtrate into a clean centrifuge tube and store it in a -80°C refrigerator
[0088] Utilizing the characteristics of the upper and lower layers of the Transwell chamber, the adipose stem cell R obtained in "Preparation Example 2" was divided into 4×10 5 The number of cells per well (cells / well) is placed in the lower 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com